1. Background
A small subset of COVID-19 patients rapidly develops acute respiratory distress syndrome (ARDS), acute respiratory failure, and other severe complications (1, 2). The virus primarily affects the respiratory system, leading to pulmonary infections (3). Studies have shown that respiratory diseases, both infectious and non-infectious, can cause an increase in pulmonary artery diameter and pulmonary hypertension (PH), depending on the extent of lung involvement (4, 5).
Among the pathophysiological mechanisms observed, two processes affecting pulmonary circulation have been identified. First, COVID-19 induces generalized endothelial inflammation (endotheliitis), including in the pulmonary microcirculation, which reduces the production of vasodilators like nitric oxide and prostacyclin by the endothelium, resulting in pulmonary vasoconstriction (6). Second, COVID-19 is associated with coagulopathy, which leads to micro- and macro-embolization in the pulmonary circulation, increasing the risk of acute PH (7). This coagulation, combined with reduced anti-platelet effects of nitric oxide and prostacyclin, causes in-situ thrombosis in small pulmonary vessels, a phenomenon rarely seen with common ICU-related diseases. These factors contribute to the development of PH and related complications.
Early detection of PH in the initial stages of the disease impacts prognosis and may require different therapeutic approaches (8, 9). Pulmonary hypertension can be diagnosed non-invasively through echocardiography, CT, and MRI (10). Acute respiratory distress syndrome, the most common cause of mortality in these cases, may also be associated with pulmonary artery microemboli and hypoxemia (11). Therefore, changes in pulmonary artery measurements during imaging are critical for the follow-up of these patients.
Parenchymal lung damage and altered pulmonary hemodynamics can lead to PH and secondary right ventricular involvement in COVID-19 patients, even in non-severe stages of the disease, as a result of hypoxic vasoconstriction in the pulmonary circulation (12). Pulmonary vascular injuries, coupled with changes in blood flow, have been identified as leading causes of right ventricular dilation and failure, contributing to COVID-19 mortality (13, 14). Moreover, patients with COVID-19 are at high risk for pulmonary embolism, particularly from severe right ventricular pressure overload (15).
Initial data suggest that the true prevalence of PH in COVID-19 patients may be around 13%, though its prognostic role remains unclear (13). Given the cardiovascular manifestations contributing to higher mortality rates in COVID-19, echocardiography may facilitate early therapeutic interventions based on imaging findings.
Chest CT scans can help detect pulmonary abnormalities and estimate disease severity in asymptomatic COVID-19 patients (16). One method to assess the severity of lung involvement in COVID-19 patients is the CT total severity score (TSS), a semi-quantitative scoring system based on the degree of pulmonary parenchymal involvement in CT scans (17). Several studies have also demonstrated a correlation between PH and changes in the pulmonary artery with the severity of COVID-19 (18).
2. Objectives
This study aims to investigate the association between the severity of lung involvement and the incidence of PH in hospitalized COVID-19 patients. Additionally, the study will evaluate the correlation between the occurrence of PH and the mortality rate.
3. Methods
3.1. Study Population
This cross-sectional retrospective study included 200 patients admitted to Ayatollah-Rohani Hospital in Babol, Iran, between March 2019 and January 2021. All patients had confirmed COVID-19 infection by PCR during hospitalization. Non-contrast chest CT scans and echocardiography were performed on all patients.
Exclusion criteria included a history of cardiovascular disease, chronic pulmonary disease, secondary hypertension, left-sided heart disease, pre-existing PH, myocarditis, connective tissue disorders such as systemic lupus erythematosus (SLE), HIV infection, portal hypertension, congenital heart disease, schistosomiasis, pulmonary venous occlusive disease, pulmonary capillary hemangiomatosis, sickle cell anemia, sarcoidosis, metabolic disorders (e.g., Gaucher’s disease), and renal diseases. The rationale for excluding these patients from the study is that these conditions are independently associated with an increased risk of PH, irrespective of COVID-19 infection (19).
The research protocol was conducted in strict accordance with the ethical principles set forth in the Declaration of Helsinki and received approval from the Ethics Committee of Babol University of Medical Sciences (reference code: IR.MUBABOL.HRI.REC.1402.015).
3.2. Computed Tomography Acquisition
High-resolution chest computed tomography (CT) scans were acquired using a 16-slice CT scanner (Siemens Medical System Inc., Erlangen, Germany). Scans were performed upon patient admission, with individuals positioned supine and instructed to maintain inspiratory breath-hold. Image acquisition proceeded in a caudocranial orientation, ensuring comprehensive coverage of the pulmonary parenchyma, extending from the apices to the diaphragmatic level (20). Any images failing to meet these acquisition parameters were systematically excluded from analysis. No intravenous contrast agent was administered. The standard CT protocol employed a tube voltage of less than 120 kVp, an automated modulation of tube current, and a low-dose CT dose index volume (CTDIvol) below 3 mGy. Scout scans were obtained from the cervical base to the superior pole of the kidneys. A board-certified radiologist with four years of subspecialty experience in thoracic imaging evaluated all scans using the INFINITT PACS system (INFINITT Healthcare Co. Ltd., Seoul, South Korea). Image interpretation was conducted using standard mediastinal window settings across axial, coronal, and sagittal planes.
To quantitatively assess the extent of pulmonary involvement, a TSS was calculated based on the presence of ground-glass opacities (GGOs), consolidation, or mixed GGO patterns within each pulmonary lobe (21). Each lobe was assigned a score from 0 to 5, corresponding to the percentage of affected parenchyma: 0 (no involvement), 1 (< 5% involvement), 2 (5 - 25%), 3 (26 - 50%), 4 (51 - 75%), and 5 (> 75% involvement). The cumulative score from all five lobes provided an individual patient’s TSS, yielding a possible range from 0 to 25.
3.3. Echocardiography
Echocardiographic evaluations were conducted using the Mindray Resona I9 ultrasound scanner (Mindray Medical International Limited, Guangdong, China), equipped with a high-frequency curvilinear transducer designed for optimal imaging resolution. The following indices were systematically extracted from the echocardiography reports:
3.3.1. Pulmonary Artery Pressure
This parameter can be estimated using the equation RVsP = PASP = 4 (peak TR velocity)² + RAP, which is applicable in the absence of pulmonary stenosis (22). In this formula, the right atrial pressure (RAP) is assessed based on the diameter of the inferior vena cava (IVC) and its percentage collapse during inspiration. Subjects with PASP > 25 mmHg were considered patients with PH (23).
3.3.2. Tricuspid Annular Plane Systolic Excursion
Tricuspid annular plane systolic excursion (TAPSE) is a scoring metric used in conjunction with non-invasive Doppler echocardiography to assess right ventricular function. It is measured by positioning an M-mode cursor parallel to the right ventricular (RV) free wall at the intersection with the tricuspid annulus, using the apical four-chamber view (24).
3.3.3. Tricuspid Regurgitation Velocity
The tricuspid regurgitation velocity (TRV) is a widely recognized parameter derived from transthoracic echocardiography (TTE) used to assess patients with suspected PH. It is estimated by visualizing the tricuspid regurgitation (TR) jet using color Doppler, acquiring its maximum velocity (TRVmax) through continuous wave Doppler (CWD), and subsequently applying this value in the simplified Bernoulli equation (ΔPRV-RA = 4 × TRVmax²) (25).
3.3.4. Right Ventricular Wall Thickness
This measurement is obtained from the parasternal long-axis view, starting at the hinge point between the aortic valve and the interventricular septum, and extending vertically towards the right ventricular endocardium (26).
3.3.5. Right Ventricular Function
The functional status of the right ventricle is categorized as either preserved or decreased (27).
3.4. Statistical Analysis
The data obtained from the study were analyzed using SPSS software version 23. Descriptive statistics were presented, including means, standard deviations, tables, and graphs. Quantitative data were analyzed using either parametric or non-parametric statistical tests, depending on the normality of the data distribution. To examine the association between pulmonary arterial pressure and the severity of pulmonary involvement, Pearson correlation analysis was employed. Additionally, to investigate the relationship between pulmonary artery pressure (PAP) and the severity of pulmonary involvement while controlling for confounding factors, linear regression analysis was performed. A significance level of less than 0.05 was considered statistically significant.
4. Results
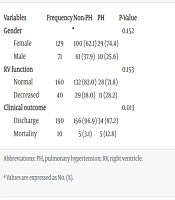
Of a total of 200 patients, 39 (19.5%) had PH. The demographic data revealed differences between the two groups. The mean age of patients with PH was 56.64 years, with a standard deviation of 14.75 years. In contrast, the mean age of patients without PH was 53.65 years, with a standard deviation of 14.21 years. As depicted in Table 1, the PH group had a higher proportion of female patients at 74.4% (29 out of 39 patients), compared to 62.1% (100 out of 161 patients) in the non-PH group; however, this difference in gender distribution was not statistically significant (P-value > 0.05). Additionally, logistic regression analysis did not identify age or the severity of pulmonary involvement as significant predictors of increased PH when their combined effects were considered (P-values for age and TSS were calculated as 0.37 and 0.33, respectively). In the PH group, the mortality rate during hospitalization was 12.8% (5 patients), compared to a mortality rate of 3.1% (5 out of 161 patients) in the non-PH group (P-value = 0.01).
| Variables | Frequency | Non-PH | PH | P-Value |
|---|---|---|---|---|
| Gender | 0.152 | |||
| Female | 129 | 100 (62.1) | 29 (74.4) | |
| Male | 71 | 61 (37.9) | 10 (25.6) | |
| RV function | 0.153 | |||
| Normal | 160 | 132 (82.0) | 28 (71.8) | |
| Decreased | 40 | 29 (18.0) | 11 (28.2) | |
| Clinical outcome | 0.013 | |||
| Discharge | 190 | 156 (96.9) | 34 (87.2) | |
| Mortality | 10 | 5 (3.1) | 5 (12.8) |
Abbreviations: PH, pulmonary hypertension; RV, right ventricle.
a Values are expressed as No. (%).
As illustrated in Table 2, CT scans of patients with PH revealed more extensive pulmonary abnormalities, with a mean TSS of 12.54 and a standard deviation of 6.36. In contrast, patients without PH showed less severe findings on CT scans, with a mean TSS of 11.44 and a standard deviation of 6.39. This difference in TSS between the two groups, averaging 1.10 points higher in lung injury severity among patients with PH, was not statistically significant (P-value > 0.05).
| Variables | Non-PH | PH | P-Value |
|---|---|---|---|
| Age (y) | 53.65 ± 14.21 | 56.64 ± 14.75 | 0.49 |
| TSS | 11.44 ± 6.39 | 12.53 ± 6.36 | 0.33 |
| TRV (m/s) | 2.29 ± 0.26 | 4.10 ± 0.65 | < 0.001 |
| PAP (mmHg) | 21.11 ± 3.66 | 34.80 ± 16.11 | < 0.001 |
| TAPSE (cm) | 1.7 ± 0.44 | 1.63 ± 0.82 | 0.88 |
| Right ventricular wall thickness (cm) | 3.26 ± 1.44 | 4.38 ± 2.82 | 0.79 |
Abbreviations: TSS, total severity score; TRV, tricuspid regurgitant velocity; PAP, pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion.
a Values are expressed as mean ± SD.
The mean and standard deviation for TAPSE in patients with and without PH were 1.63 ± 0.82 cm and 1.7 ± 0.44 cm, respectively. Additionally, the mean and standard deviation for right ventricular wall thickness in subjects with and without PH were 4.38 ± 2.82 cm and 3.26 ± 1.44 cm. None of these two indices, which indicate right ventricular function, showed statistically significant differences between the groups (P-values = 0.88 and 0.79, respectively).
In the PH group, based on TAPSE measures, 28 patients (71.8%) exhibited normal right ventricular function, while 11 patients (28.2%) had reduced right ventricular function. In comparison, the non-PH group showed better right ventricular performance, with 132 patients (82.0%) displaying normal function and 29 patients (18.0%) showing reduced function based on echocardiographic findings. Chi-square analysis indicated that this difference was not statistically significant (P-value = 0.15).
5. Discussion
The present study was conducted with the objective of investigating the relationship between the severity of pulmonary involvement and the incidence of PH in patients diagnosed with COVID-19. Although the mean CT TSS was elevated in the PH group (12.53 ± 6.36) versus the non-PH group (11.44 ± 6.39), this difference was not statistically significant.
These observations are not in accordance with several previous studies that have reported a direct association between COVID-19-related lung injury and the development of PH (28, 29). Notably, the study conducted by Wats et al. reported significantly higher TRV and PAP values in COVID-19 patients with PH compared to those without PH. Contrary to the result of our study, their investigation, which included a larger sample size of 277 patients, also highlighted an increased prevalence of PH in older patients and those with more severe disease, thereby reinforcing the connection between the severity of COVID-19 and the onset of PH (30). This discordance might be rooted in the interval between the initiation of pulmonary involvement and echocardiographic evaluation, as we performed the echocardiograms with a relatively short interval from the admission time, which might not have been adequate for the cardiac impact to appear.
Likewise, a meta-analysis by Castiglioni and Droppa underscored the high prevalence of PH in COVID-19 patients, with reported rates ranging from 16.2% to 35.3% across various studies, as well as a correlation between PH and worse clinical outcomes (13). Interestingly, our study did not identify significant differences in the mean values of age, CT TSS, TAPSE, or right ventricular wall thickness between the groups of patients with and without PH. This finding suggests that the development of PH in COVID-19 patients may not be directly related to these variables. However, it is essential to acknowledge that some studies have reported contradictory results, noting a higher prevalence of PH among older COVID-19 patients. For instance, Pagnesi et al. documented a greater incidence of PH in older patients with COVID-19, indicating an average age of 68 in the PH group compared to 58 in the non-PH group (9). The discrepancies between these findings may be attributed to differences in the studied populations, disease severity, or other confounding factors, suggesting that age could play a crucial role in the development of PH in patients with more severe manifestations of COVID-19, thereby warranting further research.
In our analysis, cross-tabulation indicated a significant association between the presence of PH and clinical outcomes, specifically discharge or mortality (P = 0.013). Patients with PH had a higher mortality rate compared to those without PH (12.8% versus 3.1%). This finding aligns with multiple studies that have demonstrated a higher mortality risk in COVID-19 patients with PH (31). For example, Wats et al. reported a significantly elevated mortality rate of 34.6% among COVID-19 patients with PH compared to 13.5% in those without (30). Similarly, Golukhova et al. identified the presence of PH as an independent predictor of mortality, with an odds ratio of 3.6 (95% CI: 1.4 - 9.4) (31).
The development of PH may contribute to increased strain on the right ventricle, leading to right ventricular dysfunction and ultimately resulting in higher mortality rates (31). Additionally, PH is associated with more severe respiratory failure and a greater need for mechanical ventilation in COVID-19 patients, which could further exacerbate the risk of mortality.
Furthermore, this study explored the potential predictive capabilities of gender and CT TSS concerning the presence of PH through multiple linear regression analysis. However, no significant predictive power was observed for these variables, aligning with findings from other studies. It is plausible that other factors, such as the clinical status of COVID-19 patients, comorbidities, or specific inflammatory markers, play a more critical role in the development of PH. Interestingly, Golukhova et al. reported a higher incidence of PH in male patients with COVID-19, which contradicts our findings (31). This inconsistency could stem from differences in the studied populations, sample size, or other confounding factors. Furthermore, the later study by Golukhova et al. included a more diverse population and adjusted for potential confounders, which may have contributed to the observed relationship between sex and PH in their research (31).
Overall, our findings suggest that, although not significantly, the mean TSS score is higher in subjects who developed PH in COVID-19 patients. This is consistent with proposed mechanisms of lung injury associated with COVID-19, such as endothelial dysfunction, inflammation, and thrombosis, which can lead to increased pulmonary vascular resistance and subsequent PH. Moreover, the presence of PH appears to correlate with higher mortality rates in COVID-19 patients, underscoring the importance of early detection and management of PH in this patient population. Pagnesi et al. emphasized that early identification of PH through routine screening with echocardiography or other non-invasive methods may facilitate timely intervention and potentially improve outcomes (9).
This study has several limitations that must be acknowledged. Firstly, the relatively small sample size of 200 patients may have restricted the statistical power to detect significant associations or differences between groups. A larger sample size is generally preferred to enhance the precision and generalizability of findings in clinical research. Secondly, the study’s single-center design may limit the applicability of the results to other populations or settings. Multi-center studies are often favored to account for potential variations in patient characteristics, disease severity, and management protocols across different healthcare facilities.
Moreover, this study did not assess the underlying mechanisms contributing to the development of PH in COVID-19 patients, such as endothelial dysfunction, inflammation, or thrombosis. A deeper understanding of the pathophysiological mechanisms could provide insights into potential therapeutic targets and strategies. Additionally, the limited follow-up duration focused solely on the acute phase of COVID-19 without evaluating the long-term outcomes or persistence of PH in affected patients. Longitudinal studies with extended follow-up periods are needed to assess the long-term implications of COVID-19-related PH, including the potential for chronic complications such as right ventricular dysfunction or pulmonary vascular remodeling.
The study also did not account for potential confounding factors that may influence the development of PH or the severity of COVID-19, including comorbid conditions, medication use, and severity markers (e.g., inflammatory biomarkers, disease severity scores). Controlling for these variables is essential for accurately interpreting the relationship between COVID-19 and PH.
Besides, the echocardiographic evaluation in this study primarily relied on measurements of TRV and PAP to assess PH. A more comprehensive echocardiographic assessment, including right ventricular function, pulmonary vascular resistance, and other hemodynamic parameters, could provide a clearer understanding of pulmonary dynamics and the severity of PH.
Lastly, the retrospective nature of this study may introduce inherent limitations associated with retrospective analyses, such as potential biases in data collection and the availability of complete and accurate medical records. In light of these limitations, future studies with larger sample sizes, multi-center designs, comprehensive echocardiographic and functional assessments, longitudinal follow-ups, and consideration of potential confounding factors are essential to enhance our understanding of the relationship between COVID-19 and PH. These investigations should also explore the long-term consequences of COVID-19 on PH and evaluate optimal management strategies.
5.1. Conclusions
In summary, this study questions the association between the severity of pulmonary involvement and the incidence of PH in patients with COVID-19. Furthermore, the presence of PH appears to correlate with higher mortality rates in this patient population. Early detection and effective management of PH in COVID-19 patients may be crucial for reducing mortality rates.