1. Background
Coronary artery disease (CAD) is the leading cause of mortality globally, resulting from several subsequent issues such as hemodynamic problems or ventricular dysfunction (1). Coronary artery disease affects left ventricular (LV) systolic and diastolic function, leading to high LV filling pressures. Validating and comparing the correlation of CAD with different LV filling pressure waveforms may be helpful in the prognosis, diagnosis, and treatment of CAD (2). Diastolic dysfunction leads to ineffective emptying of the left atrium and filling of the left ventricle, reducing the ability to increase cardiac output, increasing pulmonary pressure, and resulting in symptoms of heart failure (3). Diastolic dysfunction significantly affects mortality and hospitalization and contributes to the development of heart failure, hospitalization, and death (4, 5). Compared to systolic function, diastolic function has a longer recovery period and is more susceptible to ischemia (6).
Non-invasive assessment of LV diastolic function by left ventricular end-diastolic pressure (LVEDP) using transmitral doppler echocardiography and tissue doppler imaging provides clinicians with important information about LV diastolic function. However, both methods have relatively low sensitivity and specificity (7).
In individuals with CAD, evaluating LVEDP provides an assessment of hemodynamic status and helps guide appropriate management and therapeutic interventions (8). This parameter is assessed using various techniques, including invasive (cardiac catheterization) and non-invasive (echocardiography) approaches (9, 10). The main advantage of doppler echocardiography assessments is their ability to non-invasively calculate hemodynamic parameters. In this regard, the calculation of LVEDP, which includes early diastolic parameters, has been proposed (11). Abnormal LV diastolic filling, as assessed by transthoracic echocardiography (TTE), is associated with a worse prognosis (12, 13). Elevated LVEDP and abnormal LV relaxation have been observed in patients who underwent invasive evaluation for flow-limiting coronary artery stenosis (14, 15). To date, the correlation of non-invasively measured CAD with LVEDP has not been well evaluated. Increased ischemic load delays diastolic filling independently of clinical variables and ejection fraction (EF) (16). Indeed, subclinical atherosclerosis might induce subclinical ischemia, resulting in diastolic filling abnormalities (17, 18). It has been proposed that hypertrophy and thickening of the LV wall play a compensatory role in LV function preservation. On the other hand, diastolic dysfunction might be a non-anginal manifestation of CAD. Therefore, CAD may be a false target for treatment in patients with elevated LVEDP and consequent dyspnea (18).
2. Objectives
Therefore, our study aimed to examine the correlation between the severity of coronary artery involvement and LVEDP parameters, which may improve the understanding of the pathophysiological relationship between CAD and diastolic dysfunction.
3. Methods
In the present descriptive cross-sectional study, 90 patients were consecutively selected from those diagnosed with CAD and referred to the heart department or clinic of Modarres Hospital, Tehran, Iran, for angiography in 2023. Inclusion criteria included a diagnosis of CAD, indication for angiography, age over 18 years, and willingness to participate in the study. Patients with a history of acute myocardial infarction, congestive heart failure, hypertrophic cardiomyopathy, left ventricular hypertrophy (LVH) more severe than mild, valvular heart disease more severe than moderate, and congenital heart disease were excluded from the investigation. The study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.RETECH.REC.1402.441). The LVEDP assessment was performed using echocardiography and angiography. Echocardiographic assessment was conducted by a cardiologist and professor of echocardiography. The presence and severity of CAD were determined by an interventional specialist using cardiac catheterization. Additionally, demographic information was collected based on medical history after obtaining patient consent. Therefore, the data collection technique was observational.
3.1. Coronary Angiography
The CAD was assessed by coronary angiography (CAG) based on the maximum lumen stenosis and was defined as the presence of at least one stenosis of 50% or more in at least one of the 15 coronary segments of the three main coronary arteries (19).
3.2. Gensini Score
The severity of CAD was evaluated using the Gensini score (20). The Gensini score was calculated by assigning a severity score to each coronary stenosis based on the degree of lumen stenosis and its significance. A reduction in lumen diameter of 25%, 50%, 75%, 90%, and 99%, as well as complete occlusion, were scored as 1, 2, 4, 8, 16, and 32, respectively. Each main vascular segment was assigned a coefficient based on the functional importance of the myocardial region, including a coefficient of 5 for the left main (LM) artery, 2.5 for the proximal segment of the left anterior descending (LAD) artery, 2.5 for the proximal segment of the left circumflex (LCX) artery, 1.5 for the mid-portion of the LAD, 1 for the right coronary artery (RCA) and distal part of the LAD, the posterolateral artery, and the solitary marginal artery, and 0.5 for the other vascular segments.
3.3. Statistical Analysis
Statistical analyses were performed using SPSS software, version 22. In the descriptive section, quantitative parameters were expressed as mean and standard deviation, while qualitative parameters were presented as number and percentage. The Pearson correlation test was conducted to evaluate the correlation between data. In all tests, a P-value of less than 0.05 was considered significant.
4. Results
4.1. Patient Demographic and Clinical Characteristics
In this study, 90 patients with CAD were examined, with an age range of 36 to 83 years. Among them, 52 (57.8%) were male. The distribution of qualitative and quantitative characteristics of patients based on clinical, echocardiographic, and angiographic findings is presented in Table 1.
| Variables | Values |
|---|---|
| Qualitative Parameters | |
| Grouping (Gensini score) | |
| 1 | 47 (52.2) |
| 2 | 14 (15.6) |
| 4 | 10 (11.1) |
| 8 | 6 (6.7) |
| 16 | 10 (11.1) |
| 32 | 3 (3.3) |
| Gender (male) | 52 (57.8) |
| Comorbidities (yes) | 84 (93.3) |
| CAD (yes) | 52 (57.8) |
| Hypertension (yes) | 67 (74.4) |
| Diabetes mellitus (yes) | 26 (28.9) |
| Hyperlipidemia (yes) | 44 (48.9) |
| Smoking (yes) | 34 (37.8) |
| Familial history (yes) | 24 (26.7) |
| Quantitative Parameters | |
| LVEDP via catheterization (mmHg) | 17.16 ± 3.18 (10 - 23) |
| LVEDP via echocardiography (mmHg) | 14.75 ± 2.80 (9 - 20) |
| LVEF (%) | 56.33 ± 2.68 (50 - 65) |
| DBP (mmHg) | 73 ± 8.92 (55 - 95) |
| SBP (mmHg) | 119.83 ± 14.94 (90 - 155) |
| Age (y) | 61.63 ± 10.63 (36 - 83) |
Distribution of Qualitative Characteristics of Patients a
4.2. Correlation of LVEDP with Patients' Gensini Score
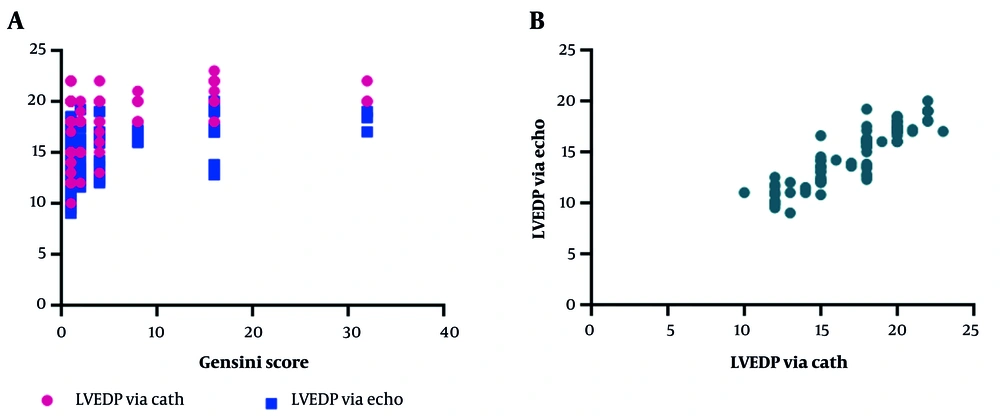
As shown in Table 2, the correlation between LVEDP and patients' Gensini scores was examined. Analyses using the Spearman test revealed a direct and weak correlation between LVEDP assessed by echocardiography and the Gensini score, which was statistically significant. There was also a significant, direct, and weak correlation between LVEDP assessed by angiography and the Gensini score (Figure 1A). Additionally, as a secondary finding, a direct and strong correlation was observed between LVEDP assessed by echocardiography and angiography (Figure 1B).
| Parameters | R Coefficient | P-Value |
|---|---|---|
| LVEDP via catheterization vs. Gensini score | 0.5 | 0.0001 |
| LVEDP via echocardiography vs. Gensini score | 0.45 | 0.0001 |
| LVEDP via echocardiography vs. LVEDP via catheterization | 0.9 | 0.0001 |
Correlation of Left Ventricular End-Diastolic Pressure and Gensini Score
5. Discussion
Diastole is an energy-dependent process and requires an adequate energy source (21). In myocardial ischemia, the energy source is decreased or becomes unavailable. Diastolic function is generally more vulnerable to injury than systolic function, meaning that diastolic dysfunction often emerges before systolic dysfunction and tends to persist longer during episodes of ischemia (22). In individuals with CAD, areas experiencing ischemia exhibit heightened myocardial stiffness. This increased stiffness, combined with a reduction in wall thinning and active pressure during ischemic events, leads to alterations in the thickness and pressure of the left ventricular wall as well as changes in the left ventricular pressure-volume relationships, ultimately raising LVEDP. As a result, CAD has a more significant effect on diastolic dysfunction (14).
In this study, we aimed to evaluate the correlation of LVEDP parameters with the severity of coronary artery involvement in CAD patients. According to the main findings, the Gensini score showed a weak, direct correlation between LVEDP assessed by echocardiography and angiography. It was also observed that there was a strong, direct correlation between LVEDP assessed by echocardiography and angiography. The occurrence of preclinical diastolic dysfunction in the overall adult population is estimated to range from 20% to 30%. This prevalence increases with advancing age and is further heightened by factors such as CAD, cardiovascular disease, and diabetes, all of which act as independent risk factors for the development of diastolic dysfunction (23).
Diastolic dysfunction describes the atypical mechanical characteristics of the myocardium and includes abnormal diastolic dilation of the left ventricle, impaired filling, chamber stiffness, and slow or delayed relaxation (24). Physiologically, any mechanism that interferes with the dissociation of the actin-myosin cross-bridge or the removal of calcium from the cytosol can delay relaxation. Individuals with impaired diastolic function are at increased risk for developing heart failure with preserved ejection fraction (HFpEF). The likelihood of progressing to HFpEF is particularly elevated for those who also suffer from conditions such as anemia, hypertension, diabetes, renal dysfunction, or CAD (25).
Left ventricular ejection fraction (LVEF), which provides a visual assessment of systolic function, has been utilized to categorize individuals recovering from myocardial infarction; however, it is not a perfect measure. Because survival rates are similar between populations with heart HFpEF and heart failure with reduced ejection fraction (HFrEF), it remains unknown whether a more integrated physiologic measure of total LV function, such as LVEDP, would better predict post-myocardial infarction heart failure. A review of three studies, including a total of 5,372 patients, found that elevated LVEDP was linked to more unfavorable outcomes after myocardial infarction, which included higher incidences of heart failure and greater mortality rates. While a lower LVEF is a well-established predictor of negative outcomes in myocardial infarction patients, those with both reduced and preserved LVEF also experience poorer outcomes, raising questions about the full effectiveness of LVEF as a predictor of long-term outcomes compared with LVEDP (26-28).
Du et al. showed that there was a significant difference in LVEDP between CAD and non-CAD groups and that LVEDP was independently associated with CAD. In different subgroups, LVEDP increased with the increasing number of occluded vessels and showed a positive correlation with the Gensini score. In the non-CAD group, LVEDP was only associated with age, not the Gensini score (29). Aging alters LV diastolic function with increased LV stiffness, increased myocardial fibrosis, decreased filling velocity and amplitude, and ultimately leads to impaired LV diastolic function (30). Therefore, clinical attention should be paid to the underlying diastolic dysfunction in the elderly.
Ren et al. conducted a study involving 693 patients with CAD and discovered that 36% exhibited mild to severe LV diastolic dysfunction. Additionally, they identified that moderate to severe LV diastolic dysfunction was a significant predictor of hospitalization due to heart failure and mortality from heart disease (31). Chronic ischemia can result in diastolic wall motion abnormalities (32). Therefore, it is important to recognize the existence of diastolic dysfunction in patients with CAD.
Lin et al. demonstrated that both obstructive and non-obstructive CAD identified through coronary CT angiography were correlated with elevated LVEDP. Their findings indicated that LVEDP rose with the increasing number of affected vessels and exhibited a positive correlation with the Gensini score, which assesses the extent and severity of CAD. This suggests that a greater burden of coronary disease may contribute to worsening diastolic function and increased pressure in the left ventricle (18). A recent investigation revealed a significant association between the degree of CAD severity and decreased LV compliance (33).
Paul et al. showed that induced LV diastolic dysfunction persists long after the resolution of the ischemic episode (34). The occurrence and degree of diastolic dysfunction are influenced by the level of ischemia. Similarly, impaired diastolic function can serve as an indicator of the severity of ischemic conditions. Perrone-Filardi et al. demonstrated that in patients with CAD, normal resting LV systolic function combined with impaired LV filling is associated with a higher likelihood of ischemia, suggesting a larger area of myocardium at risk (35). Additionally, another investigation found that patients exhibiting impaired LV relaxation tend to have more severe CAD (36).
A recent study revealed a positive correlation between LVEDP and the levels of troponin T, creatine kinase myocardial band (CKMB), and creatine kinase (CK) (37). This finding can be attributed to the fact that diastolic dysfunction of the ventricle resulting from myocardial infarction (MI) leads to an elevation in LVEDP. A study by Kirtane et al. reported an association between high LVEDP, longer hospital stays, and higher rates of heart failure 30 days after ST-elevation myocardial infarction (STEMI). Patients with LVEDP greater than 24 mmHg have been associated with a poorer prognosis and increased mortality (26). These studies indicate that LVEDP is a significant and independent predictor of readmission and prognosis in patients who have experienced a STEMI (38, 39).
Furthermore, research by Şatıroğlu et al. suggests that STEMI can lead to a decrease in left ventricular compliance, resulting in increased LVEDP and impaired left ventricular diastolic function (40). This highlights the importance of LVEDP as a prognostic indicator and its relationship with the pathophysiological changes that occur in the heart following a STEMI. In line with our study, another investigation reported that patients exhibiting high LVEDP were more likely to present with perfusion defects on nuclear scans compared to those with normal LVEDP levels (41). This finding suggests that elevated LVEDP may be associated with impaired myocardial perfusion, reflecting underlying cardiac dysfunction. Such results reinforce the notion that LVEDP can serve as an important marker for assessing coronary perfusion status and overall cardiac health in patients, particularly following events such as STEMI. This relationship further underscores the prognostic value of LVEDP in the clinical setting.
The study had some limitations. The patients studied were all clinically suspected of having CAD, which could bias the results to some extent. Additionally, there were unknown confounding factors that may affect the results. Including a control group without CAD would provide more reliable results. A comprehensive assessment of systolic and diastolic function in a large, multicenter cohort followed longitudinally with a focused echocardiographic examination in patients with CAD at all stages and severities is recommended. Furthermore, despite the attempt to examine important demographic aspects related to patients, some determinants such as the type of medication used were not considered. It is recommended that these factors be considered in future studies.
5.1. Conclusions
The present study showed that LVEDP is significantly associated with the severity and extent of CAD. Clinicians should pay attention to diastolic function in patients, especially those with CAD. These findings may have potentially important clinical and therapeutic implications. Given the high correlation between LVEDP values measured by echocardiography and angiography, LVEDP can be measured non-invasively by echocardiography before and after percutaneous coronary intervention (PCI) without any complications and can therefore be used to rapidly assess improvement in diastolic function.