1. Background
Tandem autologous hematopoietic stem cell transplantation, using high-dose cyclophosphamide and two myeloablative cycles of melphalan, is recommended for multiple myeloma treatment (1, 2). Melphalan is used for chemotherapy in the treatment of melanoma, myeloma, breast cancer, and sarcoma (3, 4).
Melphalan is an alkylating agent derived from mechlorethamine, and its mechanism of action is to inhibit DNA and RNA synthesis by forming carbonium ions, disrupting DNA replication, and crosslinking DNA strands in resting and rapidly dividing tumor cells (5). Cardiotoxicity has been found to be associated with alkylating agents such as melphalan, busulfan, carmustine, cisplatin, chlormethine, cyclophosphamide, ifosfamide, and mitomycin (6). High doses of melphalan may cause cardiotoxicity, leading to atrial fibrillation (AF) and supraventricular tachycardia (SVT) during care. The most arrhythmogenic chemotherapy agent used in ASCT is melphalan (3, 4). Due to its adverse impact on prognosis and quality of life, cardiotoxicity is the greatest problem associated with the use of various groups of chemotherapeutic agents (7). Cardiomyopathy, congestive heart failure, pericarditis, myocarditis, acute coronary syndromes, etc., are other forms of cardiotoxicity caused by cancer chemotherapy in addition to cell death (8, 9).
Dose adjustment is particularly important for patients without risk factors (history of heart disease, hypertension, diabetes, dyslipidemia, obesity). Although the risk of anthracycline-related HF is limited, patients receiving standard safe doses of anthracycline may experience heart failure after an extended follow-up period (10).
Diastolic dysfunction (DD) is critical in the pathophysiology of various cardiac conditions, including ischemic heart disease, hypertensive disease, and myocarditis, sharing a similar pathology to anthracycline-induced cardiotoxicity (11-13). The DD plays a key role in the development of heart failure, often preceding systolic dysfunction. Research has explored the effectiveness of various echocardiographic diastolic parameters in identifying anthracycline-related cardiac damage (14).
The risk of DD caused by cancer drugs must be separated from confounding factors. Many cancer patients have cardiovascular risk factors associated with DD (15, 16). The DD in cancer patients is difficult to judge because of age-related physiological changes in myocardial relaxation and the confounding effects of chemotherapy that increase cardiac workload (17, 18).
2. Objectives
Given the importance of the above, and the fact that both cancer itself and the treatments used in this disease cause heart damage, we decided to investigate DD in patients with multiple myeloma treated with melphalan using echocardiography.
3. Methods
3.1. Study Population
In this descriptive cross-sectional study, performed on 20 patients with multiple myeloma who were treated with melphalan and admitted to Ayatollah Taleghani Hospital during 2024-2025, patients under 50 years of age, without clinical cardiovascular symptoms, with normal ECG, examination, and chest radiography were included in the study. Exclusion criteria for study participants included inability to follow-up, incomplete initial data, any history of cardiovascular disease, abnormal ECG and chest radiography, murmurs and extra sounds in the heart and lungs, chronic lung disease, high blood pressure, diabetes, hyperlipidemia, smoking, and pulmonary arterial hypertension above 40 mm Hg on echocardiography. The research protocol received approval from the ethics committee at Shahid Beheshti University of Medical Sciences (IR.SBMU.MSP.REC.1403.677).
3.2. Clinical Evaluation
Patients underwent echocardiography at baseline before starting melphalan and at a 6-month follow-up. Assessments related to risk factors for heart disease, including smoking history, systolic and diastolic blood pressure, chest radiography, electrocardiography, and echocardiography, were recorded in all patients at baseline.
The person performing the echocardiography had no information about the clinical manifestations of the patients participating in the study. Various echocardiographic indices, such as wall motion abnormalities, left ventricular systolic function evaluated by ejection fraction (EF), and left ventricular diastolic function-related parameters including early diastolic velocity (E wave), late diastolic velocity (A wave), and E wave descent time (DT), were determined. Parameters related to tissue Doppler echocardiography (TDI) were determined by placing the Doppler cursor on the lateral wall and interventricular septum and determining the average of these parameters, including systolic and E wave, and examining the direction of end diastole. Additionally, by placing the Doppler cursor on specific areas of the heart, the systolic, diastolic wave velocities, and end diastolic backflow were obtained. At the same time, left ventricular systolic and diastolic volumes, stenosis and regurgitation of each valve separately, pulmonary artery pressure based on the speed of tricuspid valve regurgitation, and the presence or absence of cardiomyopathy and pericardial fluid were also evaluated. Then, changes in the mentioned echocardiographic parameters were examined in patients, and the values obtained at baseline and 6 months after the start of melphalan treatment were compared.
3.3. Statistical Analysis
Data analysis was performed using SPSS version 22 software. Quantitative variables were expressed as means with standard deviations, while qualitative variables were presented as counts and percentages. A paired t-test was employed to compare quantitative factors, with a P value of less than 0.05 deemed statistically significant.
4. Results
In this study, we evaluated 20 patients with multiple myeloma who were treated with melphalan. The age of the participants ranged from 24 to 50 years, with a mean age of 40.55 ± 11.51 years. The majority of the patients were male, comprising 60% of the cohort. Notably, there were no patients with a history of hypertension, diabetes, or smoking. The average duration of the disease among the participants was 7.6 ± 2.25 months, while the average duration of treatment with melphalan was 5.2 ± 0.89 months. These findings provide insight into the demographic and clinical profile of the patients included in the study.
4.1. Evaluation of Diastolic Cardiac Function Parameters Before and After Treatment with Melphalan
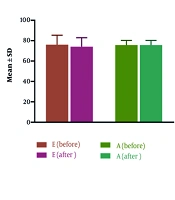
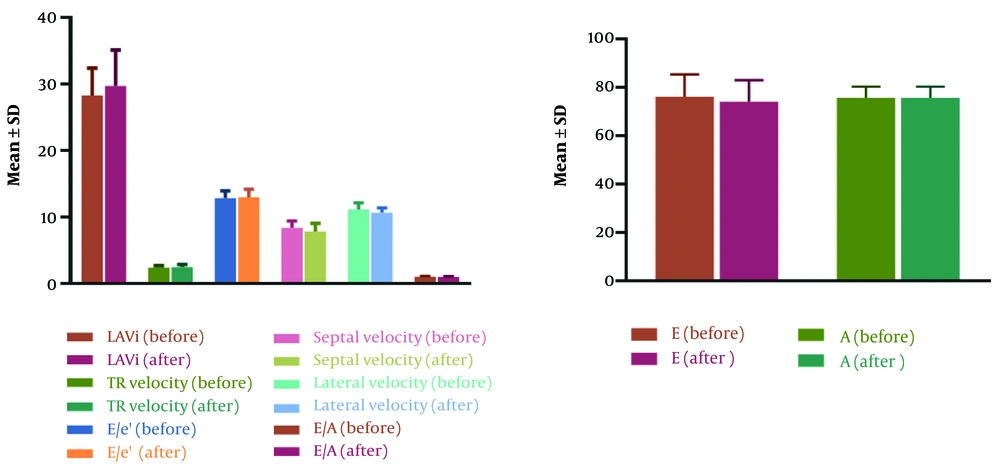
Echocardiographic parameters indicating diastolic heart function, namely, E/e’, Septal e’ velocity, Lateral e’ velocity, TR velocity, LAVi, E, A, E/A, and EF were evaluated and compared before and 6 months after treatment with melphalan. As shown in Table 1, the parameters Septal e’ velocity, Lateral e’ velocity, E/A, and E were significantly decreased after treatment. It should be noted that after treatment, it was observed that 4 patients had DD according to the criteria. The values of LAVi, E/e’, EF, TR velocity, and A did not differ significantly (Figure 1).
| Echocardiographic Parameters | At the Baseline | After 6-Month Follow-up | P-Value |
|---|---|---|---|
| LAVi (mL/m2) | 28.3 ± 4.07 | 29.7 ± 5.42 | 0.12 |
| TR velocity (m/s) | 2.37 ± 0.30 | 2.47 ± 0.38 | 0.11 |
| E/e’ | 12.87 ± 1.05 | 12.95 ± 1.22 | 0.41 |
| Septal e’ velocity (cm/s) | 8.38 ± 1.01 | 7.8 ± 1.22 | 0.008 |
| Lateral e’ velocity (cm/s) | 11.14 ± 0.98 | 11.66 ± 0.7 | 0.02 |
| E | 76 ± 9.35 | 74.1 ± 8.88 | 0.007 |
| A | 75.7 ± 4.6 | 75.6 ± 4.67 | 0.75 |
| E/A | 1 ± 0.13 | 0.98 ± 0.12 | 0.02 |
| EF (%) | 56.5 ± 5.64 | 55.25 ± 5.25 | 0.09 |
a Values are expressed as mean ± SD.
5. Discussion
Improvements in chemotherapy and radiotherapy have significantly enhanced cancer survival rates, yet their combined side effects lead to serious organic complications. Often, the combined administration of these agents leads to additive toxicities, and even when administered within the therapeutic dose range, these drugs can markedly increase their side effect profile and cause toxicity (19). The therapeutic landscape for multiple myeloma has undergone significant transformation in recent years with the advent of several new therapies and improved patient outcomes (20, 21). Melphalan, as an alkylating agent used for multiple myeloma treatment, can induce cardiac complications including SVT, AF, ventricular tachycardia, and left ventricular heart failure (22, 23). The precise mechanism by which melphalan may cause cardiotoxicity remains unclear.
In this study, 20 patients with multiple myeloma who were treated with melphalan were evaluated, who had no underlying heart disease and were under 50 years of age. According to the main results of this study, the parameters Septal e’ velocity, Lateral e’ velocity, E/A, and E decreased significantly 6 months after starting treatment with melphalan. Four patients also developed DD according to the criteria. It should be noted that the values of LAVi, E/e’, EF, TR velocity, and A did not differ significantly.
Studies on the cardiotoxicity of melphalan using echocardiography are very limited. Most studies conducted thus far have focused on a single treatment course or utilized a restricted set of laboratory and echocardiographic parameters. The majority of studies on the echocardiographic assessment of chemotherapy drugs' cardiotoxicity have been for anthracyclines. However, cardiac evaluations performed in the few studies on melphalan-treated patients were largely consistent with the results of the present study. Arrhythmias have been reported as a side effect of many chemotherapy drugs. Published studies have shown that melphalan is associated with AF in 7 - 12% of cases (71 - 73) (24, 25). A retrospective analysis showed that 11% of patients receiving melphalan before bone marrow transplantation developed SVT, of which 73% were AF or atrial flutter (3). Another study showed that a rapid ventricular rate was associated with 91.6% of patients who developed melphalan-related AF (26). Therefore, since melphalan is a mainstay of therapy for several malignancies and for bone marrow transplantation conditioning regimens, it is necessary to study the mechanism of melphalan-induced cardiotoxicity (27). To determine the potential cardiotoxicities of melphalan, a study was conducted on hiPSC-CMs by Fine and Vunjak-Novakovic Melphalan treatment of hiPSC-CMs induced oxidative stress, caused Ca2+ dysregulation and dysfunctional contractility, altered transcriptomic and proteomic profiles, and led to apoptosis and cell death. Thus, it seems that melphalan induces cardiotoxicity through the oxidative stress pathway (28).
Ma et al. documented a case involving a 58-year-old female patient with multiple myeloma who experienced sinus arrest following autologous stem cell transplantation with high-dose melphalan (29). The mechanism of AF and SVT following melphalan is unclear. Increasing age (> 60 years), larger left atrial size, higher baseline creatinine, and preexisting cardiac disease are risk factors for SVT (3). Therefore, it appears essential to monitor for cardiac toxicity in patients treated with high-dose melphalan. Zver et al. demonstrated that evident mitral regurgitation occurred in patients treated with melphalan. The lack of morphological changes in the mitral valve indicated that the regurgitation was likely a result of the drug treatment (2). There have been previous reports of mitral regurgitation after anthracycline therapy in children (30). This study suggested that patients experiencing sequential autologous HSCT are exposed to cardiac effects during the course of treatment but do not show any clinical signs of heart failure. In order to evaluate the potential late cardiotoxic impacts of sequential autologous HSCT in multiple myeloma patients, the present study included a 6-month follow-up. As expected, significant long-term changes were observed in the patients. Assessment of diastolic function is uncommon, even in clinical trials of patients treated with anthracyclines, and real-world evidence of DD is almost exclusively limited to women with breast cancer. This perspective should be expanded to include adult patients with other malignancies treated with regimens containing other drugs.
A study of breast cancer patients treated with anthracyclines showed that there was a significant number of patients in whom DD developed during chemotherapy. Ten patients (16.1%) developed DD after 3 months of treatment (31). Zuppinger et al. reported a DD rate of 20%, similar to the present study. However, this was after a longer follow-up (12 months of echocardiographic assessment). In a study, only three patients developed systolic dysfunction after three months of treatment, whereas 10 patients developed DD after three months of treatment, suggesting that LV DD appears earlier than LV systolic dysfunction (32). Standard-dose chemotherapy induces DD with preservation of LVEF in a number of cancer patients without comorbidities. These findings reinforce the hypothesis that DD is the initial sign of cardiotoxicity caused by cancer medications (33). Post-chemotherapy injuries, such as left thoracic radiotherapy or administration of the anti-Erbb2 monoclonal antibody, trastuzumab, may precipitate the progression of DD to systolic dysfunction (34). The development of comorbidities, which tend to accumulate in cancer survivors, may superimpose on DD to precipitate late cardiac events (35). Analysis of LV diastolic and systolic function is an essential part of echocardiographic evaluation, especially in cancer patients undergoing chemotherapy, as early echocardiographic changes are important for monitoring and initiating cardioprotective drugs. The present study provided important findings on echocardiographic changes in multiple myeloma patients treated with melphalan. Although many parameters of diastolic function changed significantly before and after treatment, EF did not change significantly in patients in the present study.
According to previous studies, serial measurements of LVEF do not always help identify patients at risk. In fact, LVEF may decrease when cancer drugs have caused obvious damage (36). Calabrese et al. found that asymptomatic DD with preserved LVEF was detected in 36% of patients as early as one week post-chemotherapy. The DD was observed in both a significant portion of patients receiving anthracycline-based treatments and a smaller group treated with non-anthracycline regimens (33). These findings suggested that standard doses of any common chemotherapy regimen may cause asymptomatic DD in low-risk patients. A longer follow-up period is therefore necessary to determine the clinical outcome of these patients, but DD abnormalities over time are associated with systolic dysfunction and subsequent cardiotoxicity (15). It has also been observed that patients with baseline DD are less likely to develop reduced LVEF than those who develop DD during treatment. Other studies by Upshaw and Timoteo reported that baseline DD was not associated with subsequent impairment when patients were followed for 12 months and 6.5 years, respectively (15, 37). Therefore, it seems that the evaluation of accurate echocardiographic diastolic parameters is more valuable than EF in terms of early diagnosis and timely intervention.
The E/A ratio, a standard marker for assessing left ventricular diastolic function, was significantly reduced in the present study in patients treated with melphalan during follow-up. Another study showed that the E/A ratio decreased with each successive cycle of treatment. However, more convincing evidence of left ventricular DD was provided by measuring the velocity of the right superior pulmonary vein flow, which also allows for an indirect estimate of left atrial pressure (2). In the study by Zver et al., the atrial retrograde wave (A wave) in the pulmonary venous flow increased significantly with each treatment cycle. Even more information was provided by evaluating the ratio between the duration of the mitral A wave and the duration of the retrograde A wave in the pulmonary venous flow (2). Therefore, left ventricular DD with increased left atrial pressure can be detected by the duration of the A wave and the A/a ratio. Our results indicated that DD worsened during the study period, although the A wave did not change significantly.
In complete agreement with our results, Barroso et al. observed a significant decrease in E wave velocity and E/A ratio early in treatment, while the index volume parameters AE and E/e did not change significantly (31). Similar changes in mitral inflow (decreased E/A ratio) have been reported in other studies, such as those by Ho et al. and Serrano et al. (38, 39). LAVi also did not show significant changes in the present study. LAVi, despite being an important tool for assessing remodeling and indirectly LA function, has low sensitivity in the early stages (40). In turn, changes in the E/e parameter are also associated with chronic conditions (41, 42), which explains the lack of changes in both parameters in the present study. Timoteo et al. showed significant changes in LAVi, but at a 12-month echocardiographic follow-up. First, it seems that factors such as HR and systemic blood pressure should be considered in the context of assessing diastolic function (37). Second, longer follow-up may provide a more accurate view of changes in diastolic parameters.
5.1. Conclusions
According to the results of the present study, it seems that accurate evaluation of multiple myeloma patients treated with melphalan, by echocardiography as a non-invasive and accessible method, can be useful in identifying possible early cardiac changes in these patients. Early identification of cardiac changes in favor of diastolic failure can lead to faster intervention and a better prognosis in these patients.
5.2. Limitations
One of the limitations of this study was the duration of the evaluation, which, despite showing 6-month echocardiographic changes in the study population, did not allow for an objective assessment of the longer-term behavior of systolic and diastolic function parameters related to the occurrence of cardiovascular outcomes. It would also be interesting to examine the behavior of diastolic and systolic parameters in patients undergoing additional chemotherapy with other drugs. Another important limitation is the dependence of diastolic parameters on hemodynamic conditions, such as volume depletion, a side effect associated with chemotherapy, which does not allow the conclusion that the observed changes in the E wave and E/A ratio are directly due to its cardiac toxicity.