1. Context
According to the Merriam Webster dictionary, the word “abstract” is a combination of the Latin root ab-, a prefix meaning “from” or “away,” with the verb trahere, meaning “to pull” or “to draw” and thus, it means “to make a summary”. An abstract is a self-contained, short, powerful statement that describes a larger body of work (1, 2). In scientific communications, an abstract is a tool used in a variety of contexts; it is an integral part of a published paper, book, funding proposal, thesis, research report, or a conference paper (3). In a scientific paper, the abstract is an accurate summary of the main aspects of the entire manuscript, usually in one paragraph of 150 - 300 words, using a simple, clear way of writing (4). An abstract is a truncated version of the paper that summarizes every aspect of the study (5).
The traditional form of an abstract first appeared in medical journals in the late 1950s as a descriptive paragraph at the beginning of the paper and then it became more popular (3, 6, 7). The Canadian Medical Association Journal (CMAJ) was one of the early pioneers of this practice in the early 1960s. The journal editors believed that readers did not have enough time and interest to read every paper; thus, the abstract would allow them to assess the study without actually reading the whole paper (8).
The ability to write an informative, accurate, attractive, and concise abstract is a valuable skill for researchers and writing a good abstract requires a considerable amount of time, effort, practice, mentoring, and patience (2, 3). The abstract plays a critical role in “selling” a paper to the prospective readers. A clear abstract can improve the paper search engine rankings and influence whether the user finds it and then decides to navigate to the main article (9). A well-written abstract represents a more clearly focused study, as well as the experience of the researchers (2). Despite its critical importance, the power and the role of abstracts on the pre- and post-publication success of papers are generally overlooked and they are usually written prematurely at submission time (10, 11). A badly-written and poor-quality abstract will confuse or turn off the potential readers and may also lead database indexers to make indexing errors or omissions (11).
Following our previous reports on how to write the Introduction (12), Material and Methods (13), Results (14), Discussion (15), and Title (16) of a hypothesis-testing paper, here, we provide a practical guide into writing an abstract. An overview is presented on the function, content, and organization of the abstract in a hypothesis-testing paper.
2. The Function of the Abstract
An abstract has three main functions: (1) provides a summary of the paper, (2) “sells” the paper to the editors, reviewers, and potential readers, and (3) helps with the indexing of the paper, making it retrieval by various search engines. As its name suggests, an abstract selects or pulls out the highlights from each section of the paper (17). The abstract provides a clear summary of the main story for the readers (17) and helps them to understand the main arguments of the paper quite quickly (18). An abstract generally answers at least three critical questions including “Why this study was carried out?”, “What did the authors do and how?”, and “What was the main result and what was new compared to previous works?” (19).
An abstract may critically affect both pre- and post-publication processes of the paper (11). Journal editors always read the abstract before going through the paper to get an initial impression of the work; moreover, reviewers’ decision on whether they should review a paper or not is almost entirely based on the abstract (10, 11). Although a bad abstract may not by own lead to the rejection of a paper, it does, nevertheless, pave the way for a negative response of the editor (11). The abstract motivates the readers to go through the main text as it is the main mechanism by which readers decide on whether they should obtain and read the full paper (4).
The abstract is also used for indexing purposes, as most databases enable readers to search abstracts and to have a quick retrieval that limits the extraneous items recalled by a “full-text” search (18). A poorly-written abstract may lead to indexing errors or make the paper inaccessible in a literature search (11).
3. Content of the Abstract
The abstract of a hypothesis-testing paper consists of four basic parts: (1) the study question/hypothesis/objective, (2) the experiments/methods conducted to answer the question, (3) the results of the study, and (4) the answer to the question (7, 17). Furthermore, the abstract may start with some background information, which will put the current study into perspective (20). The abstract may, then, end in applications, implications, recommendations, or speculations based on the answer to the question (17). The basic elements of the abstract of a hypothesis-testing paper are listed in Table 1.
| Section | Details |
|---|---|
| Background | 2-3 sentences of background information to highlight the importance of the study question/hypothesis: (1) What is already known (2) What is unknown (knowledge gap or problem) |
| Study question/hypothesis | What the study intended to examine |
| The experiments/materials and methods | Study design (e.g. observational, interventional), randomization, blinding, placebo control, criterion standards for diagnostic tests |
| Setting: The level of care, e.g. primary, secondary, etc. | |
| Materials/subjects/participants: (1) The materials studied (i.e. molecule, cell, tissue, or organ) (2) The animal/human participants (number, sex, species or ethnic group if appropriate, clear definition of selection, inclusion and exclusion criteria) (3) The condition of the animals or subjects (if necessary) | |
| Intervention (if any): What, how, when, where | |
| The main outcomes | |
| Results | Selective and most important results that answer the question (i.e. specific effect sizes and their statistical and clinical significance, if possible) |
| Answer to the question | Statement of the answer to the question |
| Importance of the findings | A sentence stating the application, recommendation, implication, or speculation based on the answer |
3.1. Background (Introduction)
The background information should be brief and in harmony with that given in the introduction section of the paper (17). Similar to the introduction, the background starts with a general topic (what is known in the field) and knowledge gap or problem, and narrows down to a specific topic (study question/hypothesis) of the study (4) (for more details see (12). The beginning can be more interesting by creating stress, e.g., by making a statement followed by “however” or “but” and then “stating a problem”, “contradiction”, or “gap in knowledge” (7). Addressing the author’s previous work in the background section of the abstract makes it annoying (4).
3.2. Hypothesis/Question
Study question/hypothesis or objective is a clear statement of the main aim of the study and major hypothesis tested or research question posted (21). Without addressing the study question/hypothesis, the abstract is meaningless and lacks an anchor for understanding the methods or the results (22). For questions including both an independent variable (X) and a dependent variable (Y), the question should be stated clearly using a verb to relate the independent and dependent variables, e.g., to determine whether X causes Y (17). For questions with only a dependent variable, the specific aspect of the dependent variable studied must be stated (17).
3.3. Experiments/Materials and Methods
To address the materials and methods used, essential and more important details are enough to indicate “how the hypothesis was tested” (4, 17), including design, setting, subjects/participants, interventions (if any), the main outcome (s) (7, 21), and a brief description of statistical methods (4). The experimental approach or the study design, including both independent and dependent variables, is also needed (17). When authors address the study setting, they need to give general rather than specific information (e.g., instead of naming the center, they can give the geographical location if it is important) (21). Describing standard techniques such as ELISA, PCR, etc. should be avoided (4). If the methodology is unique or of interest, addressing the methodological aspects of the study may be appropriate (2).
3.4. Results
Not all results, but only the most pertinent (those answering the question) are presented in the abstract (4, 17). The main findings should be presented, not as general and broad statements but as specific results/data and their statistical significance (absolute numbers, percentages, means, coefficients, ratios, P values, confidence intervals) (2, 23). A common flaw in abstracts is the inclusion of P values without providing the data; P values alone are not useful (17). Giving a P value should be accompanied by the mean, standard deviation, and sample size (17). Provide percent change rather than actual values (e.g. mean and standard deviation) when a quantitative idea of the data is approached (4, 17). To make the abstract more efficient, details of the experiment (e.g., duration of the study, dependent variables) may be included in the statements of the results (17). Referring to data that are presented later in the manuscript should be avoided (4).
The results presented in the abstract should be arranged in a logical order, including chronological order and importance order (most-to-least or least-to-most important) (17). In the most-to-least important order, the experimental results come first and the control results are presented last. Similarly, variables that have changed come before variables that did not change (17). Another logical order is that the details of the results be presented in the same order as the details in the study question (17); for example, if the question is “whether lesions of the nucleus tractus solitarius alter pulmonary artery pressure and pulmonary lymph flow without altering the systemic circulation”, so the results can be organized in the same order, first pulmonary artery pressure, next pulmonary lymph flow, and last systemic circulatory variables (17).
The study groups should be named clearly, e.g. intervention or controls. If baseline/pretreatment characteristics of the study participants are similar between the groups, there is no need to show all of them for each group; overall key median or mean values would suffice with a statement NS, i.e. non-significant (24).
3.5. Answer and Its Importance/Conclusion(s)
The answer to the question should be supported by data and must not go beyond the data presented (4). In this section, the authors need to state whether the hypothesis is accepted or rejected based on the data presented (4). The conclusions should be straightforward, brief, and specific to the study findings/observations (24). If word limit permits, the conclusion may begin with an opening statement such as “Our study showed …” or “Our results indicated…”. New and important aspects of the study or observations need to be emphasized (23). The answer should not be just a restatement of the results and no data should be presented here (4). To answer the question, use the same key-terms, point of view, and verb as in the question (17).
In the final sentence, state the importance of the work, e.g., if the conclusion (s) leads to change (s) in concept or the understanding of the field (4). This can be presented by stating the applications, recommendations, implications, or speculations that are based on the findings (17). Expressing the importance of the work should not be replaced with the answer to the question (17). Try to avoid any broad/general statements about the need for more research (2); instead, give explicit recommendations for further studies if warranted (15). Authors are advised to be specific and focused on their findings, do not overestimate the importance of them, and avoid broad claims and strong statements since even pioneering breakthrough studies require independent confirmation (24).
3.6. Others
The International Committee of Medical Journal Editors (ICMJE, www.icmje.org) recommends that, if applicable, journals should include the clinical trial registration number at the end of the abstract (25). Furthermore, funding sources are also proposed to be listed separately after the abstract to facilitate proper display and indexing for search retrieval by MEDLINE (26).
It is suggested that abstracts do not include figures, tables, or citations to previous works (17). If authors are convinced that the abstract must include a reference to significant previous work, they should give the full reference because the abstract will stand alone in abstracting publications (27).
4. Organization of the Abstract
Similar to the text of the paper, an informative abstract is organized in the following order: background (if any), question, experiments, results, answer to the question, and importance of the work (by stating applications, recommendations, implications, or speculations) (17). Journals may favor an unstructured abstract, which is just a conventional abstract with running text; or they may prefer a more structured format that has distinct labeled sections (28). Historically, because almost all published papers did not provide any essential details in their abstracts, Ertl and Gazette in 1969 proposed that for all medical, clinical, and experimental papers, the important contents should be presented in a tabular format (29). After several revisions (30, 31), “a more informative” abstract for articles of medical/clinical journals was defined with subheadings for background, objective, design, setting, participants, interventions (if any), outcomes, results, and conclusions (28). In 1993, ICMJE recommended the use of structured abstracts (23). The percentage of published papers in medical journals containing structured abstracts increased from 2.5% in 1992 to 20.3% in 2005 (32) and this number rose to more than 30% in 2010 (33).
Compared to the traditional format, structured abstracts provide more details, with clear headings for the main components of the abstract (30, 31, 34). This format also enables the readers to quickly judge about applicability and validity of the findings for clinical practice (30). Structured abstracts are also easier to search and more simple to read, and are generally welcomed by readers and authors (35). The structured abstract, however, has been criticized for its greater length and its imposed style and rigid uniformity that may inhibit author creativity and may bore the reader (27).
To organize a structured abstract, a factual standard reflecting the process of scientific discovery i.e. “Introduction-Methods-Results-Conclusions” is commonly recommended by medical journals (e.g. New England Journal of Medicine, The Lancet, Archives of Internal Medicine, American Journal of Medicine) (36, 37). Other patterns of subheadings are also recommended, e.g., the 8-heading format proposed by Haynes et al. (30); a more frequent non-IMRAD (Introduction, Methods, Results, and Discussion) format (37) is also used by some journals (e.g., BMJ, Journal of American Medical Association, Annual Review of Medicine). The ICMJE does acknowledge that the format of structured abstracts may differ amongst journals (25). Many reporting guidelines now recommend specific abstract formats depending on the study design, such as systematic reviews and randomized trials (28, 38, 39).
5. Features of a Well-Written Abstract
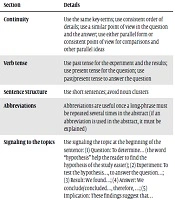
The ICMJE recommends that the abstract should emphasize new and important aspects of the study or observations, and not overinterpret the findings (25). A good abstract is simple, specific, clear, unbiased, honest, concise, precise, complete, and (preferably) structured (40). Since readers may never read further than the abstract, it should provide a general understanding of what was studied, how the study was done, what was found, and what conclusions were drawn. A well-written and informative abstract stands on its own, apart from the rest of the manuscript (4, 17). It, however, should be consistent with the main text and exhibit the key message (s) of the paper (40). An important feature of a well-written abstract is the following of a consistent story or keeping continuity, defined as moving smoothly from the background information to the conclusion (17). Of course, a good abstract must be based on data already collected and analyzed. Reading abstracts from recent issues of the target journal may also provide some helpful hints (2). Some general tips to write an effective abstract are provided in Table 2.
| Parameter | Details |
|---|---|
| Continuity | Use the same key-terms; use consistent order of details; use a similar point of view in the question and the answer; use either parallel form or consistent point of view for comparisons and other parallel ideas |
| Verb tense | Use past tense for the experiment and the results; use present tense for the question; use past/present tense to answer the question |
| Sentence Structure | Use short sentences; avoid noun clusters |
| Abbreviations | Abbreviations are useful once a long-phrase must be repeated several times in the abstract (if an abbreviation is used in the abstract, it must be explained) |
| Signaling to the topics | Use signaling the topic at the beginning of the sentence: (1) Question: To determine… (the word “hypothesis” help the reader to find the hypothesis of the study easier); (2) Experiment: To test the hypothesis…, to answer the question…; (3) Result: We found…; (4) Answer: We conclude/concluded…, therefore, …; (5) Implication: These findings suggest that… |
| Length | Follow the author guidelines (usually 250 words or less is recommended). Some strategies for shortening an abstract: (1) Eliminate meaningless phrases; (2) Eliminate phrasal verbs and superlatives; (3) Cut prepositions, especially “of”; (4) Change noun phrases to verbs |
6. The Procedure for Writing an Effective Abstract
The abstract is written after completing all experiments and interpreting the data (4). Writing an abstract requires careful, logical, and clear thinking. To draft an abstract, a stepwise process needs to be followed (28). Planning, drafting, reviewing, peer-reviewing, editing, and packaging are proposed as essential steps of developing an abstract (2, 41). Overall, the initial step is to consider the manuscript entirely and select key contents, weight the importance of each word, and iteratively polish the story (28). In drafting an abstract, a practical and efficient suggestion is to copy and paste from the main text. Thus, 2 - 3 key sentences can be selected each from the introduction, material and methods, and discussion (mainly the first or the concluding paragraph), and several sentences from the results (including statistical analysis) (28). Next, the obtained unfocused and disorganized text needs to be extensively edited by removing unnecessary details and extra words to provide coherence and a natural flow (28).
The first draft is proposed to be set aside for 1 - 2 days (a short resting period) and then, the authors need to edit it again; they can send it for peer review by an unbiased outsider (e.g., a colleague, advisor, other mentors) to give thoughtful, concise, and honest criticism of the work (2). After careful consideration of the comments, the authors can promote their work and prepare the final draft (2). The final step that needs to be considered is packaging, which is done by following the journal style and a final check for possible misspelled words, incorrect grammar, exceeding the word count, and failure to comply with size and font specifications (2).
7. Most Common Flaws and Mistakes in Writing an Abstract
Taking a look at the common mistakes and flaws of the published abstracts (extensively discussed elsewhere (11, 17)) is helpful to make an effective abstract. In brief, these weaknesses include omitting or vaguely stating the question, stating an application/implication instead of an answer, and substituting a descriptive abstract for a hypothesis-testing study (17). Missing important information, exceeding the word limits, providing extraneous information (e.g., literature findings around the topic), lacking appropriate organization, and overstating the data are other common mistakes that are generally seen in poorly written abstracts (11). Apart from content mistakes, there are also two other common mistakes that are generally made in writing an abstract for a scientific paper. These are formal aspects (e.g., the layout of the abstract, its structure and length) and linguistic-stylistic aspects (grammar and spelling, stylistics and punctuation) (42). The typical characteristics of a poorly-written abstract include not being self-sufficient, being like an introduction rather than a summary, containing irrelevant details, and not giving any background information (19). Other common flaws include misleading reporting, misleading interpretation and inadequate extrapolation of the results, using causal language, linguistic spin, inadequate statement of implications for clinical practice, and absence of negative results (43). Some common flaws and mistakes in writing an abstract are provided in Box 1.
| Common Flaws |
|---|
| Providing too general, too much, or not enough background information |
| Using the same sentence for the first line of the abstract and the first line of the introduction |
| Stating claims that are not supported within the paper |
| Holding back important information to try to get the reader to go through the paper |
| Using terms that are too technical or too generic |
| Using generic quantification (e.g., many, several, few) |
| Using words and phrases that add no value, like vague expressions and abstract nouns (refer to intangible things, like actions, feelings, ideals, concepts, and qualities) |
| Over and unjustified use of subjective adjectives (e.g., innovative, interesting, fundamental, challenging, vital, cutting-edge) |
| Providing unnecessary details |
| Including too many or not enough methods |
| Using abbreviation, jargon, and other language shortcuts that may lead to confusion |
| Not following the instructions to authors provided by the target journal |
| Not articulating the hypothesis, rationale for the study, sample size, and conclusions |
8. Abstract for a Scientific Meeting
The selection of presentations in scientific meetings is based on abstracts (4) and the main function of a meeting abstract is, therefore, to showcase the author’s valuable contribution and to highlight the work for attracting audiences (10, 17). Writing a meeting abstract needs to follow the same guidelines as abstracts for papers, except that it is likely to include more details of the methods and to display data in a table or a graph (17). More details regarding writing an effective and informative abstract for a meeting are presented elsewhere (17, 45, 46).
9. Keywords
At the end of the abstract, 3 to 10 keywords or short phrases are usually provided that are used for cross-indexing so that various search engines can retrieve the paper. Keywords are proposed to be obtained through the Medical Subject Headings (MeSH) list of Index Medicus (23). It is suggested that keywords be different from the words that are within the main title; however, they can be variants of the terms/phrases that are used in the title, the abstract, and the main text (40). Effective keywords are those that are familiar within the field and are specific to the paper (i.e., terms used more than twice in the text) (10). Listing very general terms as keywords is not recommended (e.g., protein or DNA) because they are not helpful (10). A practical guide to choosing effective keywords is to list the main related keywords and then, doing a search using the same words to verify whether they are effective in retrieving appropriate papers within the field of interest (10). Authors can also use the “MeSH on-demand” browser for selecting keywords (https://meshb.nlm.nih.gov/MeSHonDemand).
10. Conclusion
Overall, a well-written abstract should accurately summarize the main aspects of the full paper. It should be simple, clear, unbiased, honest, concise, precise, stand-alone, complete, and preferably structured. The first impressions that an abstract makes may go a long way towards the decisions made by the editors and the reviewers of the paper. Also, the post-publication success of the paper, such as citation performance, is also affected by the abstract. The ability to make an informative and accurate abstract, including a concise and clear statement of the problem/gap of knowledge, the motivation behind the research, the study question/hypothesis, enough description of the experiments, novel results, and a captivating conclusion, is a critical skill with broad implications. Authors, therefore, need to follow available guidelines and journal’s guide for authors to arrange a strong and convincing abstract.