1. Context
Levothyroxine (LT4) monotherapy has been considered the standard treatment for hypothyroidism. The results of the 2017 American Thyroid Association (ATA) survey in 389 members showed that as many as 18% - 41% of physicians might add liothyronine (LT3) therapy while reducing the LT4 dose and 9% - 29% would add LT3 therapy while maintaining the LT4 dose in their practice (1). This was in contrast to another comprehensive survey reported in 2013, which found that 8.8% of physicians routinely used combined LT4-LT3 therapy compared to 3.6% who used such therapy in patients with symptoms of hypothyroidism (2). Physicians practicing in North America were more likely to use combination therapy.
Although both 2012 and 2014 ATA guidelines concluded that there is insufficient evidence to support the routine use of LT3 medications (3, 4), approximately one-third of physicians in the 2017 survey expressed their willingness to prescribe therapies other than LT4 monotherapy. The ATA survey finding is in line with some reports from other countries that the LT3 use is rising in the care of patients with hypothyroidism. Sales of LT3 increased by six folds in Denmark between the first and the last quarter of 2013. Combination therapy is prescribed by general physicians. Those on treatment are in particular middle-aged, well-educated women, with 85% of responders describing positive effects and dose adjustment being performed by about a quarter of patients themselves (5). In some countries, patients demand combination therapy; physicians, in turn, may feel threatened because their explanation of why they are reluctant to add LT3 is not accepted by patients, often in an aggressive manner (6).
Before 1970, the majority of patients with hypothyroidism were being treated by natural preparations of thyroid extracts, containing both T4 and T3 hormones (7). Growing discontent with variable potency of thyroid extract products (8), along with the decreased cost of LT4, caused an increase in the prescription of LT4 (9). Many physicians hesitated to use LT4 monotherapy because they believed that the ideal thyroid hormone preparation should have a combination of LT4 and LT3 to simulate the metabolic effects of endogenous thyroid secretion (10, 11). However, the ability of the human body to generate T3 in hypothyroid patients on LT4 therapy, as documented in the classic study by Braverman et al. (12), obviated this concern. Assuming that LT4 monotherapy maintains the satisfactory serum pool of T4 and the process of deiodination provides T3 availability to tissues has made LT4 therapy the standard replacement therapy in hypothyroidism (4, 13).
In recent years, the belief that restitution of euthyroidism is accomplished in all hypothyroid patients by LT4 monotherapy has become doubted (14-17). Scholarly articles related to LT4+LT3 combination treatment increased in 2018 - 2019, and there has been much enthusiasm for continued research to answer the question as to whether or not LT4-LT3 combination is beneficial (and superior to LT4 monotherapy) for treatment of hypothyroidism (18-21).
This review aimed to investigate the pros and cons of LT4 monotherapy versus LT4+LT3 combination therapy and to explore current knowledge regarding combination therapy by LT4 and slow-release T3 (SR-T3) preparations.
2. Evidence Acquisition
Two authors searched PubMed and Scopus using search terms “hypothyroidism” AND “levothyroxine” OR “thyroxine” OR “liothyronine” OR “triiodothyronine” OR “LT4” OR “T4” OR “T3” OR “LT4+LT3” OR “T4+T3” OR “combination therapy”. The related articles were selected and confirmed by all authors.
3. Results
3.1. Levothyroxine Monotherapy
It has been reported that many hypothyroid patients on LT4 monotherapy complain of specific symptoms of hypothyroidism (22). Morreale de Escobar et al. documented that euthyroidism could not be restored in plasma and all tissues of thyroidectomized rats following T4 monotherapy (23). Other studies showed that patients treated with LT4 had significant impairment in psychological well-being (14) and lower resting energy expenditure compared to healthy controls (16) while demonstrating lower and even suppressed serum TSH concentrations (15).
Studies employing sensitive TSH assays have shown that adequately treated hypothyroid patients with LT4 have higher serum total T4 and free T4 (fT4) and lower T3:T4 ratios than controls (24, 25). In LT4-treated patients, the relationship between decreased T3:T4 ratios and abnormal variables is not yet fully understood.
Saravanan et al. found no correlations between General Health Questionnaire-12 (GHQ-12) or the thyroid symptom questionnaire and serum T3 concentrations in LT4-treated patients (26). In 2011, Gullo et al. documented that LT4 monotherapy could not guarantee euthyroidism in athyreotic patients, as these patients had highly heterogeneous T3 production capacity during LT4 monotherapy, abnormal T3:T4 ratios were seen in > 20% of them (15). In another study, resting energy expenditure (REE) and body composition were normal in TSH-suppressed participants, but there was abnormally low REE in LT4-treated euthyroid women, associated with lower mean free T3 (fT3) levels (16). Based on NHANES data, compared to healthy individuals, those on LT4 had lower serum fT3:fT4 ratios and different metabolic variables. The female sex and serum creatinine had negative, and body mass index (BMI), total cholesterol, and triglycerides had positive relationships with serum fT3:fT4 ratios. However, only were a few clinical characteristics significantly associated with the fT3:fT4 ratio (17). A meta-analysis of 99 studies also showed increased serum LDL-C and triglyceride concentrations in LT4-treated participants (27). It has also been shown that the serum levels of fT4, T3, and TSH, as well as BMI and lipid profiles, in euthyroid Graves’ patients differ from those of patients taking LT4 (28), confirming previous ideas that the consumption of exogenous LT4 cannot mimic all physiological actions of normal thyroid (16, 27); the explanation is that in euthyroid subjects, the serum levels of thyroid hormones are determined by their thyroid secretion, as well as T3 production from T4 in peripheral tissues via deiodinases pathways. In contrast, in LT4-treated patients, the only origin of circulating T3 is T4 deiodination.
In those treated with LT4 monotherapy, high serum T4 concentrations inhibit type 2 deiodinase (D2) activity, which results in lower T3 content in D2-expressing tissues such as the brain. The LT4 therapy decreases whole body D2-dependent conversion of T4 into T3, while D2 activity in the hypothalamus is minimally affected by T4 (29). Therefore, the serum TSH levels may be in the reference range by slightly higher fT4 concentrations, while the conversion of T4 into T3 is low in peripheral tissues, leading to lower serum and tissue T3.
3.2. LT4+LT3 Combination Therapy
The LT4+LT3 combination therapy in patients with hypothyroidism resulted in the increased serum concentrations of both T4 and T3 and increased fT3/fT4 ratio (22, 30). This ratio may be more, equal to, or less than the ratio in healthy subjects, depending on the prescribed LT4 and LT3 dosage.
Does LT4+LT3 combination therapy perform superior to LT4 monotherapy in the normalization of symptoms in hypothyroid patients? Most studies have shown that despite almost normal fT3/fT4 ratios, the outcome of combination therapy was not superior to LT4 monotherapy. Neither baseline serum T3 nor changes in serum T3 during treatment could predict the response (31). Therefore, there is a lack of evidence as to whether low serum T3 concentrations or fT3/fT4 ratios are linked to persistent symptoms.
The LT4+LT3 combination therapy may affect some of the thyroid hormone-dependent actions. The LT4+LT3 combination therapy may result in decreases in serum cholesterol and increases in the activation of bone resorption (32). In those taking T4 monotherapy, patients with mildly suppressed TSH levels appear to be close to euthyroidism, whereas those with normal serum TSH commendation may exhibit peripheral tissue hypothyroidism (33). There exists some evidence that during LT4+LT3 therapy, the normal TSH concentration is accompanied by the euthyroid state in various tissues (29).
Although more patients prefer LT4-LT3 combination therapy over LT4 monotherapy and patients on combination therapy may lose more weight (22), one systematic review (34) and one meta-analysis (35) showed that outcomes including fatigue, mood, anxiety, depression, and quality of life were not better in LT4+LT3 therapy than in LT4 monotherapy. In genetic studies, the association of polymorphisms in thyroid hormone transporters and deiodinases with a preference for LT4+LT3 combination therapy has not been well clarified (36).
Regarding the appropriate LT4-LT3 dose ratio, values between 13:1 and 20:1 have been suggested based on the physiologic secretion ratio of T4:T3 by the thyroid gland (18, 22).
There is an enormous impact of LT4+LT3 combination therapy on daily clinical practice. A large number of patients on LT4+LT3 therapy in some western countries with probable cardiac and skeletal adverse reactions (4) along with the response of major professional societies that T4 monotherapy remains the standard treatment for hypothyroidism indicate a dilemma in clinical practice. However, the European Thyroid Association has a weak recommendation based on low-quality evidence about the use of LT4-LT3 aiming to help patients with persistent complaints despite normal TSH in LT4-treated hypothyroid patients: “It is suggested that L-T4 + L-T3 combination therapy might be considered as an experimental approach in compliant L-T4-treated hypothyroid patients who have persistent complaints despite serum TSH values within the reference range, provided they have previously given support to deal with the chronic nature of their disease and associated autoimmune diseases have been ruled out” (22).
3.3. LT4+Slow Release LT3 Combination
During the last century, physicians worked at replacing hormone deficiency with the hormone that is missing, mainly using thyroxine, cortisol, growth hormone, gonadotropins, and sex hormones (37). However, hormone replacement therapy for various endocrine gland deficiencies faces difficulties due to variations in hormone absorption, serum binding, and transfer, cellular receptors, peripheral conversion, and metabolism. Besides, tissue and pathway-specific biomarkers are not well recognized, and those already known may not be entirely sufficient for a complete understanding of the cellular effects of hormones (38). In the case of LT4 therapy, in addition to the above limitations, some hypothyroid patients receiving LT4 replacement therapy do not feel as well as matched controls (15, 22). The combination of LT4 and LT3 could not achieve all objectives of optimum replacement therapy because LT3 that is rapidly absorbed into the bloodstream has a short half-life of approximately one day and may result in unwanted non-physiologic serum peaks (39). Therefore, it has been proposed to substitute LT4 therapy for hypothyroidism with slowly absorbed LT3 preparation with a carefully determined ratio relative to LT4 (40). New methods for LT3 delivery, including slow-release tablets, liquid formulations, T3-hybrid molecules such as T3 sulfate, poly-zinc-T3, and glucagon-T3, nanoparticles containing T3, subcutaneous implants of T3-containing matrices, and stem cells for de novo development of the thyroid gland have been suggested or developed (41).
Hennemann et al. reported a six-week trial with a combination of 125 μg LT4 and 6 μg Slow Release (SR) T3 (in-house slow-release preparation) in hypothyroid patients. The T3 levels showed delayed Tmax and lower Cmax and serum fT4/fT3 during LT4+SRT3 therapy compared to LT4 monotherapy; however, the ratio was still higher than in controls (42). There were no differences in serum T4 or TSH between the two groups. In addition, there was a plateau in serum T3 after SRT3 preparation ingestion between two and nine hours and a decline at nine hours afterward, indicating that for a convenient regimen, the drug must be administered at least twice daily.
The LT4 and slow-release LT3 formulations with different compositions have not been confirmed in any other clinical trial or have had minimal effects on Cmax and Tmax. The details of new products are not available due to commercial interests.
3.4. Future Directions
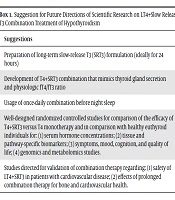
Although much progress has been reported in the formulation of SR-T3 preparations (41), no further clinical study has been reported on T4+SRT3 combination therapy for hypothyroidism. Optimally, many scientific investigations need to be conducted (Box 1). First, the SRT3 formulation that can release T3 for more than 12 hours (ideally 24 hours) in the intestine for absorption should be developed. Second, the combination of T4+SRT3 for a once per day dosage needs to be developed, which hopefully mimics the molar ratio of T4/T3 secreted by the thyroid gland (43). Third, such preparation may be more conveniently taken before sleep at night to mimic the midnight T3 surge in normal individuals. Fourth, such a hitherto magic preparation should be used in multiple well-designed randomized clinical trials to evaluate the long-term efficacy and safety of LT4-SRT3 combination therapy. These studies should be directed toward serum hormonal concentrations and tissue- and pathway-specific biomarkers and identification of potential predictors for effective LT4+SRT3 combination therapy using genomics and metabolomics data from population-based cohorts. Studies should also assess symptoms and quality of life and identify patient characteristics that are associated with this parameter. Finally, the safety of LT4+SRT3 administration in patients with cardiovascular disease and the effects of prolonged therapy of this formulation on cardiovascular and bone health should be determined.
| Suggestions |
|---|
| Preparation of long-term SRT3 formulation (ideally for 24 hours) |
| Development of LT4+SRT3 combination that mimics thyroid gland secretion and physiologic fT3/fT4 ratio |
| Usage of once-daily combination before night sleep |
| Well-designed randomized controlled studies for comparison of the efficacy of LT4+SRT3 versus LT4 monotherapy and in comparison with healthy euthyroid individuals for: (1) serum hormone concentrations; (2) tissue and pathway-specific biomarkers; (3) symptoms, mood, cognition, and quality of life; (4) genomics and metabolomics studies. |
| Studies directed for validation of combination therapy regarding: (1) safety of LT4+SRT3 in patients with cardiovascular disease; (2) effects of prolonged combination therapy for bone and cardiovascular health. |
Suggestions for Future Directions of Scientific Research on LT4+SRT3 Combination Treatment of Hypothyroidism
4. Conclusions
The beneficial effect of LT4+LT3 combination therapy is not clear, and the safety of long-term therapy is yet under question. In countries where multiple products of T3 are available and public interest has been directed toward the uncertain effects of T3 and T4+T3 combination therapy, tensions between patients and physicians are on the rise. More scientific well-designed research projects are required in this field, in particular in the formulation of optimal T4+SRT3 and careful evaluation of the product, aiming at optimal therapy for hypothyroidism.