1. Background
When the assay to measure the serum thyroid-stimulating hormone (TSH) level was not sensitive enough to differentiate a suppressed level in Graves’ hyperthyroidism (GD) from a normal level in healthy control, the T3 suppression test measuring the thyroidal radioactive iodine uptake (RAIU) after administering T3 (1) and TRH loading test measuring the serum TSH level after TRH injection (2) were used to confirm the diagnosis of GD or clarify the possibility of remission. The restoration of pituitary responsiveness was reported to occur 2 - 5 weeks after the withdrawal of thyroid-suppressive therapy with T4 in normal subjects (3). However, GD patients frequently have persistent TRH unresponsiveness after euthyroidism has been achieved by antithyroid drug (ATD) treatment, suggesting the early relapse of GD if ATD is withdrawn (4-6). Following the introduction of the sensitive TSH assay (7), continued suppression of the serum TSH level was attributed to the TSH receptor antibody (TRAb) activity (8-10).
TSH-binding inhibitor immunoglobulin activity (TBII) or TRAb was found to be associated with the pathogenesis of GD (11, 12) and could be a better marker for the activity of GD (6, 13, 14). Although a better prognosis was suggested when the early disappearance of TBII was observed (15, 16), a long-term follow-up study for more than eight years suggested that the disappearance of TBII within two years did not indicate a better prognosis than in patients who were found with TBII disappeared after two to five years. However, the prognosis of these patients was far better than that of the patients with TBII remained positive for more than 5 years (17).
When the serum TSH level is suppressed (8-10) or TBII is positive (6, 12-14), the possibility of remission is low. However, the relapse rate of GD is 25% - 26% even when the response to TRH is normal (5) or TBII becomes negative (14), probably due to fluctuating-type GD, which has recently been reported in detail (17).
2. Objectives
In this retrospective study, the changes in the serum-free T4 (fT4) and TSH levels in the early stage of ATD treatment were compared with the long-term changes in the serum TBII activity to evaluate the relationship between the early thyroid and pituitary response to ATD and the long-term prognosis.
3. Methods
The clinical course of 609 patients with untreated GD who were initially treated with 15 mg of MMI in our hospital between 1981 and 2001 (18, 19) was retrospectively evaluated. The diagnosis of GD was made based on elevated serum fT4 and/or fT3 levels, a suppressed serum TSH level, and TBII positivity and/or thyroid-stimulating antibody (TSAb) positivity. A diffuse high thyroidal RAIU was confirmed in all patients (20). The patients were initially treated with 15 mg of MMI, followed by tapering or titration, mainly depending on the changes in serum fT4 and fT3 levels. When the serum fT4 level remained high, the MMI dosage was increased to 20 - 30 mg. When patients exhibited signs suggestive of adverse effects, the drug was changed to propylthiouracil or potassium iodide.
As to the early response to ATD, the clinical course for 180 days after the initiation of the treatment was classified depending on the changes in serum fT4 and TSH levels (Table 1).
| Group | Comparison of the Clinical Data Before Treatment | ||||||
|---|---|---|---|---|---|---|---|
| A1 | A2 | B1 | B2 | B3 | C | P Value | |
| Serum TSH | Elevated | Normalized | Remained Suppressed | ||||
| Serum-Free T4 | Became Low | Normalized | Became Low | Normalized | High fT3 | Remained High | |
| No. (%) | 48 (7.9) | 188 (30.9) | 31 (5.1) | 185 (30.4) | 84 (13.8) | 73 (12.0) | |
| Clinical data | < 0.0001 | ||||||
| Age, y | 41.3 ± 17.0 | 37.9 ± 14.6 | 40.6 ± 13.7 | 36.2 ± 13.5 | 35.7 ± 12.2 | 29.1 ± 11.0 | < 0.0001 |
| Sex (male:female) | 16:32 | 52:136 | 7:24 | 34:151 | 14:70 | 16:57 | 0.1277 |
| AntiTg Ab+, % | 22/46 (47.8) | 67/186 (36.0) | 11/31 (35.5) | 70/181 (38.7) | 23/84 (27.4) | 22/72 (30.6) | 0.0085 |
| Anti MC Ab+, % | 37/46 (80.4) | 138/186 (74.2) | 23/31 (74.2) | 141/181 (77.9) | 65/84 (77.4) | 58/72 (80.6) | 0.7052 |
| Free T4, ng/dL | 5.4 ± 3.9 | 6.5 ± 3.7 | 9.5 ± 9.8 | 7.7 ± 5.1 | 10.4 ± 6.4 | 8.9 ± 5.8 | 0.2062 |
| Free T3, pg/mL | 13.5 ± 6.9 | 15.0 ± 7.7 | 20.4 ± 13.2 | 16.9 ± 8.5 | 22.7 ± 19.6 | 17.9 ± 6.3 | 0.4710 |
| Tg, ng/mL | 41 (26 - 139) | 62 (20 - 144) | 91 (28 - 255) | 72 (21 - 148) | 130 (74 - 224) | 100 (30 - 167) | 0.6802 |
| TBII, % | 39.1 (26.0 - 62.9) | 33.9 (22.0 - 50.8) | 59.2 (31.8 - 80.2) | 46.7 (27.1 - 68.7) | 62.0 (35.8 - 76.6) | 52.1 (32.6 - 70.8) | 0.1253 |
| TSAb, % | 275 (136 - 1029) | 185 (119 - 309) | 623 (321 - 1243) | 271 (127 - 540) | 287 (148 - 900) | 260 (139 - 432) | 0.0080 |
| Thyroid vol., mL | 26.4 (18.5 - 39.8) | 25.5 (18.5 - 35.1) | 33.6 (26.1 - 46.3) | 30.1 (23.0 - 46.1) | 40.0 (31.8 - 54.6) | 36.8 (30.0 - 48.0) | 0.0114 |
| RAIU, %/5 h | 49.8 (31.9 - 69.4) | 47.6 (28.8 - 67.1) | 69.0 (53.7 - 76.4) | 57.9 (41.1 - 71.9) | 60.6 (45.7 - 72.1) | 64.5 (40.5 - 75) | 0.6738 |
Abbreviations: Anti MC Ab, antithyroid microsomal antibody; AntiTg Ab, antithyroglobuin antibody; RAIU, thyroidal radioactive iodine uptake; TBII, TSH-binding inhibitor immunoglobulin; Tg, thyroglobulin; TSAb, Thyroid-stimulating antibody; Thyroid Vol., estimated thyroid volume.
aValues are expressed as mean ± SD, or median (IQR), or No. (%).
bGroup A1; serum TSH level became elevated with low fT4; Group A2; serum TSH level normalized with normal fT4; group B1, serum fT4 level became low, but TSH level remained suppressed (inappropriately suppressed serum TSH); group B2, serum fT4 and fT3 levels normalized but TSH level remained suppressed; group B3, serum fT4 normalized but fT3 level remained high with suppressed TSH level (T3 toxicosis pattern); group C, serum fT4 level remained high (refractory), and serum TSH level remained suppressed in 373 (61.2%) patients.
Since the serum MMI level was not monitored in our hospital, complete adherence to ATD was not assured in this retrospective study.
Regarding the change in TBII, patients who were followed for > 5 years were classified into three types (17): smooth type, TBII was negative before the treatment or positive TBII became negative in 5 years; if TBII became positive again in smooth type, the patients were re-classified as fluctuating type. Fluctuating type was further divided into two types: early relapse type, if an exacerbation was observed when the patient was still taking ATD or within one year after the withdrawal of ATD; and late relapse type, if an exacerbation was observed more than one year after the withdrawal of ATD (13). Cases that remained TBII-positive for > 5 years were classified as smoldering type.
The long-term prognosis was evaluated in 527 patients who were followed for > 2 years. If a patient remained euthyroid with normal serum TSH level and negative TBII for > 1 year after the cessation of the drug, they were considered to have entered remission. If the patient seemed to be almost in remission with normal fT4, TSH, negative TBII, and an impalpable or small goiter but wanted to continue ATD therapy with a small maintenance dose, the patients were classified into the possible remission patients. Otherwise, they were classified into non-remission patients. Patients who became spontaneously hypothyroid were classified as hypothyroid patients.
The thyroid function was evaluated, as previously reported (17). The reference values in our laboratory were as follows:
Serum fT4 = 0.8 - 1.7 ng/dL, fT3 = 2.2 - 3.8 pg/mL, TSH = 0.42 - 3.81 mU/L, thyroglobulin (Tg) < 30 ng/mL, TBII < 15%, TSAb < 180%, RAIU 2% - 18%/5 h, anti-Tg or antithyroid microsomal antibody measured by haemagglutination < 100 dilution.
3.1. Statistical Analyses
Statistical analyses were performed using the JMP 14 software program (SAS Institute, Inc., Cary, NC, USA). Normal continuous variables were expressed as the mean ± standard deviation, while median and interquartile ranges were reported for skewed variables. Comparisons were made using multivariate logistic regression analysis. The differences in the distributions of categorical variables were confirmed by the chi-square test. P values of < 0.05 were considered to indicate statistical significance.
The present study was approved by the Ethics Committee of Kyushu University. We did not obtain informed consent from the participants involved in our study because the study was restricted to existing data with all personal identifiers removed.
4. Results
4.1. Classification of the Response to MMI During the First 180 Days Depending on the Changes in the Serum TSH and fT4 Levels
Following the administration of 15mg of MMI during the first 180 days, 48 patients (7.9%) became hypothyroid with elevated serum TSH level (A1) and 188 patients (30.9%) became euthyroid with normal fT4 and TSH levels (A2) (Table 1). The MMI dosage was then tapered. On the other hand, the serum TSH level remained suppressed, whereas the serum fT4 level became low in 31 patients (5.1%) (B1-inappropriately suppressed TSH) or the serum fT4 and fT3 levels normalized in 185 patients (30.4%) (B2). The MMI dosage was reduced depending on the changes in the serum fT4 or fT3 levels even if the TSH level remained suppressed to avoid MMI-induced iatrogenic hypothyroidism, which might exacerbate exophthalmos. The serum fT3 level remained high, whereas the serum fT4 level normalized in 84 patients (13.8%) (B3-T3 toxicosis). Finally, the serum fT4 level remained high in 73 patients (12.0%) (C-refractory). Thus, the serum TSH level remained suppressed in 373 patients (61.2%) during the first 180 days.
Clinical course and the long-term prognosis were shown in Tables 2 and 3, respectively.
| Group | Comparison of the Clinical Data During Treatment-Abnormal Data Observed (Days) or the Duration of Treatment, y | ||||||
|---|---|---|---|---|---|---|---|
| A1 | A2 | B1 | B2 | B3 | C | P Value | |
| Serum TSH | Elevated | Normalized | Remained Suppressed | ||||
| Serum-free T4 | Became Low | Normalized | Became Low | Normalized | High fT3 | Remained High | |
| I | Improvement of Serum Thyroid Hormone and TSH Levels | ||||||
| No. (%) | 48 (7.9) | 188 (30.9) | 31 (5.1) | 185 (30.4) | 84 (13.8) | 73 (12.0) | |
| Free T4, days | 31 (21 - 45) | 41 (25 - 59) | 41 (21 - 67) | 56 (33 - 104) | 66 (35 - 98) | 297 (224 - 469) | |
| Free T3, days | 57 (38 - 77) | 65 (41 - 98) | 68 (32 - 115) | 83 (47 - 131) | 333 (226 - 624) | 368 (264 - 654) | |
| TSH, days | 80 (68 - 101) | 95 (69 - 137) | 454 (244 - 812) | 364 (239 - 663) | 451 (307 - 863) | 668 (376 - 1573) | |
| II | Pattern of Changes in Serum TSH-Binding Inhibitor Immunoglobulin (TBII) Level During Follow-Up of > 5 Years (N = 416) | ||||||
| No. (%) | 30 (100) | 135 (100) | 24 (100) | 115 (100) | 62 (100) | 50 (100) | |
| Smooth typeb | 11 (36.7) | 64 (47.4) | 6 (25.0) | 59 (51.3) | 25 (40.3) | 17 (34.0) | |
| Fluctuating typec | 12 (40.0) | 58 (43.0) | 8 (33.3) | 35 (30.4) | 22 (35.5) | 15 (30.0) | 0.0044 |
| Smoldering typed | 7 (23.3) | 13 (9.6) | 10 (41.7) | 21 (18.3) | 15 (24.2) | 18 (36.0) | |
| III | Improvement of Clinical Parameters and the Treatment Period | 0.0053 | |||||
| TBII, days | 342 (175 - 1055) | 244 (117 - 503) | 657 (238 - 2494) | 500 (200 - 1015) | 488 (312 - 1237) | 717 (280 - 1639) | 0.0039 |
| Struma, days | 355 (88 - 1079) | 444 (156 - 1545) | 2335 (418 - 3581) | 859 (364 - 2582) | 2037 (831 - 3915) | 1658 (583 - 3135) | 0.0922 |
| Withdrawal of ATD, y | 3.2 (1.8 - 8.0) | 5.7 (2.0 - 9.7) | 11.0 (6.2 - 12.7) | 5.7 (3.0 - 10.1) | 6.7 (3.4 - 11.4) | 6.2 (3.6 - 10.2) | 0.4708 |
| Follow-up, y | 12.0 (2.7 - 19.6) | 12.9 (4.2 - 22.7) | 10.1 (5.4 - 22.3) | 8.8 (2.4 - 23.4) | 13.7 (4.8 - 22.5) | 12.0 (2.8 - 21.2) | 0.6140 |
Abbreviation: ATD, antithyroid drug.
aValues are expressed as mean ± SD, or median (IQR), or No. (%).
bTBII became negative in < 5 years and remained negative.
cTBII became negative in < 5 years but became positive again.
dTBII remained positive for > 5 years.
| Group | A1 | A2 | B1 | B2 | B3 | C | Total | P Value |
|---|---|---|---|---|---|---|---|---|
| Serum TSH | Elevated | Normalized | Remained Suppressed | |||||
| Serum-free T4 | Became low | Normalized | Became low | Normalized | High fT3 | Remained High | ||
| I | Long-Term Prognosis of the Patients Followed for > 2 Years When Including Ablated Patients (N = 527) | |||||||
| No. (%) | 38 (100) | 170 (100) | 29 (100) | 156 (100) | 75 (100) | 59 (100) | 527 (100) | |
| Remissionc | 17 (44.7) | 78 (45.9) | 7 (24.1) | 73 (46.8) | 31 (41.3) | 17 (28.8) | 223 (42.3) | |
| Possible remissiond | 8 (21.1) | 28 (16.5) | 5 (17.2) | 16 (10.3) | 8 (10.7) | 4 (6.8) | 69 (13.1) | 0.0182 |
| Non-remission | 12 (31.6) | 54 (31.8) | 16 (55.2) | 64 (41.0) | 31 (41.3) | 35 (59.3) | 212 (40.2) | |
| Spontaneous hypothyroid | 1 (2.6) | 10 (5.9) | 1 (3.4) | 3 (1.9) | 5 (6.7) | 3 (5.1) | 23 (4.4) | |
| II | Ablated Patients (N = 111) | |||||||
| Ablation n (% in total) | 6 (15.8) | 28 (16.5) | 10 (34.5) | 27 (17.3) | 19 (25.3) | 21 (35.6) | 111 (21.1) | 0.0128 |
| Ablation, y | 5.7 (2.9 - 10.2) | 9.3 (5.5 - 14.0) | 5.3 (3.0 - 8.3) | 4.5 (1.6 - 9.6) | 7.0 (1.8 - 10.9) | 3.5 (1.9 - 8.5) | 5.9 (2.3 - 10.9) | 0.0419 |
| III | Long-Term Prognosis of the Patients Followed for > 2 Years After Excluding Ablated Patients (N = 416) | |||||||
| No. (%) | 32 (100) | 142 (100) | 19 (100) | 129 (100) | 56 (100) | 38 (100) | 416 | |
| Non-remission | 6 (18.8) | 26 (18.3) | 6 (31.6) | 37 (28.7) | 12 (21.4) | 14 (36.8) | 101 (24.3) | 0.2019 |
aValues are expressed as mean ± SD, or median (IQR), or No. (%).
bSince 111 patients were treated by ablative therapy and were branded as non-remission patients even if they might have the chance of remission after the long ATD treatment (II), long-term prognosis was re-evaluated after excluding the ablated patients (III). Percent of non-remission patients were 40.2% when including ablated patients (I) and 24.3% after excluding ablated patients (III). Therefore, the non-remission rate would be 24% - 40% during ATD treatment without ablative therapy.
cThe patient remained euthyroid for > 1 year after the cessation of the antithyroid drug (ATD) and TBII remained negative.
dThe patient seemed to be almost in remission with normal free T4, TSH, and negative TBII but wanted to continue ATD with a small maintenance dosage.
4.2. Three Patterns of Changes in Serum Level of TBII in Patients Followed for > 5 Years
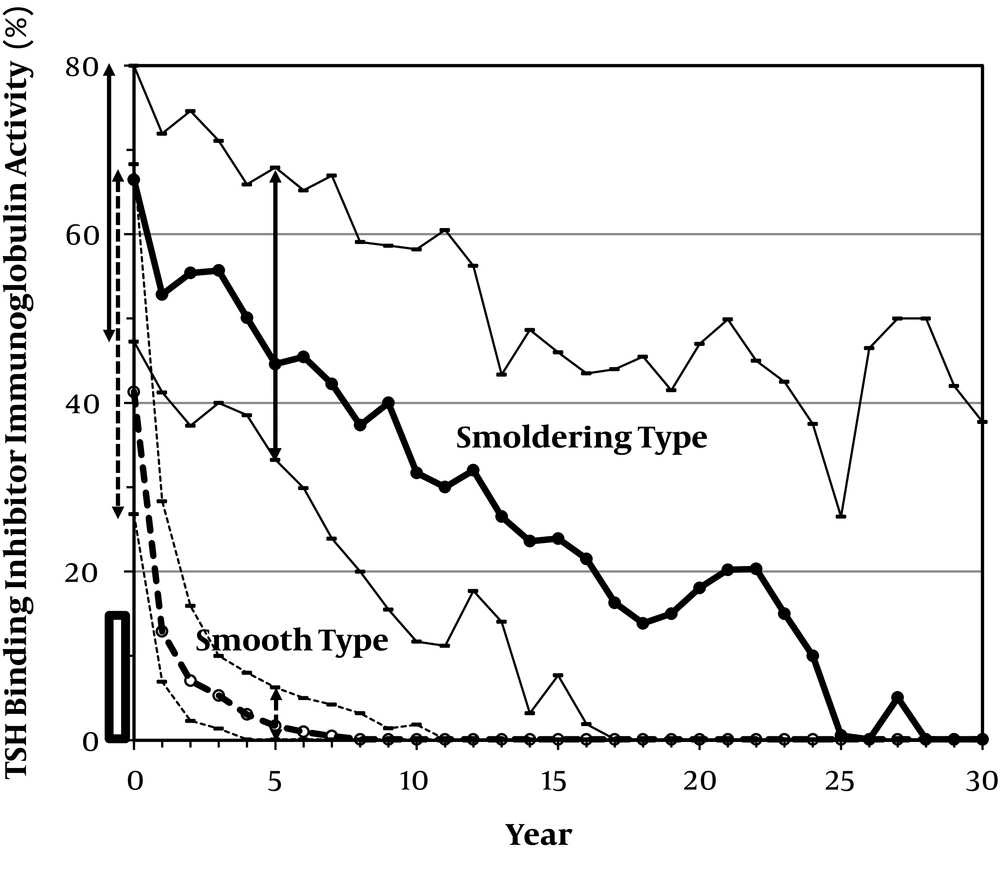
The pattern of TBII change (17) was evaluated in 416 patients who were followed for > 5 years. Before treatment, TBII was negative in 41 (9.9 %) of the patients, despite high RAIU. Among the other patients, TBII became negative within 5 years in 291 patients (70.0%). Thus, negative TBII was observed within 5 years in 332 patients (79.8%). Among these 332 patients, TBII remained negative in 182 patients (54.8%), suggesting a smooth type (Figure 1). In contrast, TBII became positive, suggesting fluctuating type in 150 patients (45.2%) while they were taking an ATD (n = 124) (early relapse) or long after the cessation of the drug (n = 26) (late relapse). Therefore, of the 416 patients who were followed for > 5 years, 182 patients (43.8%) were smooth type, and 150 patients (36.1%) were fluctuating type, as shown in Table 4.
The changes in the serum TSH-binding inhibitor immunoglobulin (TBII) activity, measured using a first-generation radioreceptor assay kit (Baxter Health Care Co., Ltd., Tokyo, Japan) (normal range < 15%), during the long-term follow-up of Graves’ hyperthyroid patients who were initially treated with 15mg of methylmercaptoimidazole (MMI). Cases, in which TBII was negative before therapy or TBII-positive cases, in which the patient became negative within five years and remained negative (smooth type) and cases, in which the patient remained TBII-positive for > 5 years (smoldering type) are shown (median and interquartile range). Ranges before treatment and after five years of treatment are also shown by a solid line for smoldering type and broken line for smooth type, suggesting apparent difference after five years of treatment.
| Smooth Type | Fluctuating Type | Smoldering Type | P Value | |
|---|---|---|---|---|
| I (Initial Clinical Data) | < 0.0001 | |||
| No. (%) | 182 (43.8) | 150 (36.1) | 84 (20.2) | |
| Age, y | 39.2 ± 14.7 | 33.3 ± 12.2 | 37.0 ± 15.6 | < 0.0001 |
| Sex (male:female) | 37:145 (M20.3%) | 27:123 (M18.0%) | 13:71 (M15.5%) | 0.9121 |
| AntiTg Ab+, % | 63/181 (34.8) | 54/149 (36.2) | 30/84 (35.7) | 0.5423 |
| AntiMC Ab+, % | 142/181 (78.5) | 113/149 (75.8) | 63/84 (75.0) | 0.4795 |
| Free T4, ng/dL | 8.1 ± 5.4 | 7.2 ± 4.8 | 8.8 ± 7.6 | 0.8856 |
| Free T3, pg/mL | 18.2 ± 8.3 | 16.7 ± 8.9 | 19.6 ± 19.6 | 0.8192 |
| Tg, ng/mL | 86 (25 - 160) | 69 (23 - 134) | 125 (38 - 205) | 0.1184 |
| TBII, % | 45.8 (28.5 - 68.3) | 36.4 (23.7 - 57.5) | 69.1 (54.2 - 78.7) | < 0.0001 |
| TSAb, % | 230 (135 - 425) | 234 (128 - 473) | 489 (183 - 1266) | 0.6158 |
| Thyroid Vol., mL | 32.1 (22.7 - 45.3) | 30.6 (21.8 - 42.1) | 38.2 (28.5 - 48.1) | 0.4324 |
| RAIU, %/5 h | 58.7 (39.3 - 74.3) | 55.4 (37.3 - 69.6) | 58.3 (38.6 - 71.5) | 0.1383 |
| II (Comparison of the Clinical Data During Treatment-Abnormal Data Observed, d) | < 0.0001 | |||
| Free T4, d | 50 (28 - 86) | 48 (28 - 102) | 63 (32 - 140) | 0.6367 |
| Free T3, d | 87 (49 - 176) | 98 (45 - 178) | 122 (48 - 357) | 0.7084 |
| TSH, d | 230 (99 - 528) | 215 (86 - 470) | 505 (188 - 1190) | 0.0086 |
| Struma, d | 1032 (240 - 2051) | 1163 (293 - 3677) | 2450 (865 - 4289) | 0.0026 |
| TBII, d | 391 (155 - 750) | 335 (171 - 658) | 3789 (2389 - 6342) | |
| TSAb, d | 194 (0 - 576) | 184 (0 - 571) | 1414 (285 - 3458) | |
| III (Long-Term Prognosis) | 182 (100) | 150 (100) | 84 (100) | |
| Remission | 146 (80.2) | 47 (31.3) | 12 (14.3) | |
| Possible remission | 13 (7.1) | 6 (4.0) | 5 (6.0) | < 0.0001 |
| Non-remission | 15 (8.2) | 87 (58.0) | 62 (73.8) | |
| Spontaneous hypothyroid | 8 (4.4) | 10 (6.7) | 5 (6.0) | |
| Ablation | 11 (3.7) | 50 (29.1) | 38 (45.2) | < 0.0001 |
Abbreviations: Anti Tg Ab, antithyroglobulin antibody; anti MC Ab, antimicrosomal antibody; T4, thyroxine; T3, triiodothyronine; TBII, TSH-binding inhibitor immunoglobulin; TSAb, thyroid-stimulating antibody; RAIU, radioactive iodine uptake; d, days.
aValues are expressed as mean ± SD, or median (IQR), or No. (%).
TBII remained positive for > 5 years of continuous therapy in 84 patients (20.2%), suggesting smoldering-type. However, it was noteworthy to find that TBII values continuously decreased during 30 years of follow-up (Figure 1). Among these patients, 38 patients (45.2%) were treated by ablative therapy (radioiodine therapy or thyroidectomy). In the other 46 patients, TBII remained positive in 21 patients and became negative in 25 patients, 7.3 (6.1 - 20.5) years after the initiation of the therapy. This resulted in remission in 12 patients, possible remission in 5 patients, and spontaneous hypothyroidism in 5 patients. In the other 3 patients, TBII became positive again and continued ATD therapy, suggesting the presence of a fluctuating course, even in the smoldering type.
4.3. Clinical Findings of the Patients with Different Initial Responses to MMI
The clinical data before treatment revealed that the refractory patients (group C) were younger and that the patients in groups B and C had a larger thyroid gland (Table 1). The prevalence of anti-Tg antibody-positive patients was higher in group A1, and the TSAb titer was higher in group B1. There were no significant differences in the GD activity, including the serum fT4, fT3, Tg, TBII levels, and RAIU.
4.4. Comparison Between the Response to MMI Treatment During the First 180 Days and the Change in the Serum TBII During the Clinical Course or the Long-Term Prognosis
Among the patients who were followed for > 5 years, smoldering type TBII change was more frequently found (Table 2, Part II) and the time required for the normalization of serum TBII was longer (Table 2, Part III) in groups B1 and C than in groups A1, A2, B2, or B3. There was no difference in the time required for the disappearance of struma or the time required to withdraw ATD (Table 2, Part III).
Among the patients who were followed for > 2 years, non-remission was more frequently observed in group B1 patients with inappropriately suppressed TSH (55.2%) or group C patients with an intractable clinical course (59.3%) (Table 3, Part I).
In total, remission was observed in 223 patients (42.3%), possible remission was observed in 69 patients (13.1%), non-remission was observed in 212 patients (40.2%), and spontaneous hypothyroidism was observed in 23 patients (4.4%) (Table 3, Part I). Among the patients with different prognosis, there was no significant difference in the disease severity before treatment (e.g., RAIU or serum fT4, fT3, and TBII levels) (data not shown). The time required for the first disappearance of TBII was 960 (286 - 8596) days in the non-remission patients, and 333 (151 - 714) days in the remission patients, a significant difference was observed (P = 0.0003).
Considering the fluctuation of TBII, a small maintenance dose of ATD was carefully continued for approximately 4.6 (1.6 - 8.4) (median, interquartile range) years even after the disappearance of TBII. Remission of GD occurred after 6.2 (3.0 - 10.4) years of treatment.
4.5. Clinical Findings of the Patients with Different TBII Change Types and Long-Term Prognoses
Table 4 shows the clinical findings of patients with different patterns of serum TBII change. The fluctuating type was frequently observed in younger patients. The initial TBII value was higher (Table 4, Part I) and the time required for the normalization of the serum TSH level and for the struma to become impalpable was much longer in smoldering type (Table 4, Part II); however, there were no significant differences in disease severity before treatment, including the serum levels of fT4, fT3, and RAIU (Table 4, Part I). The time required for the normalization of both the serum TBII and TSAb levels differed according to type. Remission, including possible remission, was eventually observed in 159 patients (87.3%) with the smooth type (Table 4, Part III). These patients, who accounted for 38.2% of the 416 patients who were followed for > 5 years, were considered to meet the definition of the smooth remission. In contrast, remission, including possible remission, was observed in 53 (35.3%) of the 150 patients with the fluctuating-type, and only in 17 (20.2%) of the 84 patients with the smoldering type.
After excluding patients with spontaneous hypothyroidism, remission was not achieved in 15 patients (8.2%) with the smooth type, 87 patients (58.0%) with the fluctuating-type, and 62 patients (73.8%) with the smoldering type (Table 4, Part III). Among these 164 non-remission patients, 65 patients (39.6%) continued ATD and 99 patients (60.4%) were treated with ablative therapy.
As shown in Tables 3 and 4, approximately 4% - 6% of the patients, mainly women (males: females = 1:22), in each TBII change type, became spontaneously hypothyroid without ablative therapy. The change in TBII activity was variable and the patients became hypothyroid even when they were still positive for TBII. Five patients were positive for blocking type TBII.
4.6. Influence of the Ablative Therapy on the Calculated Non-Remission Rate During ATD Treatment
As shown in Table 3, Part II, ablative therapy was performed in 111 (21.1%) of the patients, which was approximately half of the patients in non-remission patients, 2 - 11 years after the initiation of ATD therapy. Ablative therapy was performed more frequently in groups B1 and C (about 35%) than in groups A1, A2, B2, or B3 (15% - 25%) and much earlier in group C than in the other groups. After excluding these ablated patients, there was no significant difference in the percentage of non-remission patients among the groups (Table 3, Part III). Percent of non-remission patients were 40.2% when including ablated patients (Table 3, Part I) and 24.3% after excluding ablated patients (Table 3, Part III).
5. Discussion
The popular ATD treatment protocols for GD is to continue ATD for 12 - 18 months and discontinue it once the TBII levels have normalized while continuing ATD or switching to definitive therapy (radioactive iodine or surgery) if the TBII level remained elevated (21, 22). However, whether a 12- to 18-month interval is appropriate remains unclear (23, 24). Recently, Azizi et al. (25) reported increased remission rates after long-term MMI therapy for 60 - 120 months. In comparison with this “time to treat” method, there are alternative “treat to target” methods without fixed-duration therapy. Namely, the patients are advised to continue ATD treatment until GD is no longer present, regardless of the time taken to achieve this end (13, 17, 26-29); however, the issue with this approach is that it is difficult to tell when patients have achieved remission.
Approximately 28 years ago, Ikenoue et al. (13) reported that the combination of thyroid stimulation factors (TBII, goiter size, serum Tg, and RAIU) could be a useful marker for identifying a relapse of GD after ATD was stopped. If more than 3 factors were positive, early relapse occurred in 100% within a year. However, even if all factors were negative, remission was observed in 86% of the patients after the withdrawal of ATD, early relapse occurred in 10% and late relapse occurred in 4%, suggesting the difficulty in predicting the remission of GD (13).
In the present study, we confirmed the poor prognosis of the GD patients if TBII remained positive for > 5 years (smoldering type) or TBII became positive again during the clinical course (fluctuating type) (Table 4) (17). Regarding the changes in serum TSH, it was surprising to find that the serum TSH level remained suppressed in 63% of the patients during the first 180 days (Table 1). The most striking group was B1, in which serum TSH remained suppressed despite a low fT4 level. The percentage of patients with smoldering TBII was higher not only in group C (intractable patients) but also in group B1 (Table 2, Part II). The time required for the normalization of serum TSH in the smoldering type patients was significantly longer in comparison with the smooth TBII disappearance type (Table 4, Part II). Thus, it was suggested that inappropriate suppression of serum TSH or poor recovery of the hypothalamus-pituitary-thyroid axis during ATD treatment might suggest persistent TBII activity (8-10).
This study also suggested that even if patients show a refractory response to the initial treatment with 15mg of MMI, they still have a 35% chance of remission and a 5% chance of becoming spontaneously hypothyroid (Table 3, Part I). It was also suggested that remission could be expected even in 35% of fluctuating-type or 20% of smoldering-type patients if they were followed for > 10 years with ATD treatment (Table 4, Part III). The tenacious continuation of low-dose ATD therapy is considered to be safe (26, 30) for patients who wish to avoid radioactive iodine or surgery.
Regarding the long-term prognosis, we must consider the effect of ablative therapy. After ablation, the patients would be branded as non-remission patients, and early ablative therapy made the remission rate low (31). An interesting finding was that the difference in the long-term prognosis among the patients with differing early responses to MMI treatment became ambiguous after excluding ablated patients (Table 3, Part III). In our present study, the non-remission rate was 40.2% when including ablated patients (Table 3, Part I) and 24.3% after excluding ablated patients (Table 3, Part III). Therefore, remission or spontaneous hypothyroidism was suggested to be likely in 60% - 75% of the GD patients if they had been treated with ATD for more than 10 years without ablative therapy. In other words, approximately a quarter of the patients may have found difficulty achieving remission, even after a long-term follow-up. They require continuous ATD treatment or could be treated by radioactive iodine.
The strength of this study is a long-term follow-up of a large number of GD patients but the study is limited by the fact that about 30% of the patients dropped out during the course.
In conclusion, prolonged suppression of serum TSH may suggest active TBII activity during treatment, and continuous TBII positivity for more than five years suggests persistent GD activity, although there is still the possibility of remission following long-term ATD treatment.