1. Context
Graves’ Disease (GD) is the most common cause of hyperthyroidism in iodine-sufficient areas around the world. It is an autoimmune disease where a class of immunoglobulin G is detected in the circulation of patients along with thyroid-stimulating antibodies (TSAb). These antibodies are a clonal response to a TSH receptor-related antigen on the plasma membrane of thyroid follicular cell antibodies against thyroid receptors and are called TSH receptor antibodies (TRAb). Thyroid-stimulating antibodies act by stimulating thyrotropin receptors and causing excess production and release of the thyroid hormone, leading to hyperthyroidism. These antibodies can also block thyrotropin receptors, resulting in hypothyroidism. The stimulatory and blocking antibodies’ actions seem to regulate the level of thyroid function (1).
The first description of the disease was made more than 180 years ago, but its three treatment options have remained the same for the last 75 years: antithyroid drug (ATD), radioiodine, and surgery. These three modalities have advantages and disadvantages. In the absence of specific treatment and permanent cure, the aim of treatment is only to control hyperthyroidism, which can be achieved by ATD. Radioiodine and surgical resection of the thyroid are considered definitive treatments. Hypothyroidism is the goal when these treatments are used; therefore, L-thyroxine replacement is mandatory (2-4).
Antithyroid Drugs, including methimazole, carbimazole (a methimazole analog), and propylthiouracil, can inhibit the synthesis of the thyroid hormone and may have direct effects on autoimmunity. They are the first choices in Europe, Asia, and Latin America (2). Even in the United States, where radioiodine has been the first choice for decades, recent studies now suggest that ATD is the most common modality of treatment (3).
If ATD is chosen as the primary therapy, treatment should last for 12-18 months. Thyroid-stimulating antibodies should be measured after this period, and if titers are negative, ATD may discontinue. If remission is not obtained, low doses of methimazole may be used according to patient preference (4), or definitive treatment should be utilized. The advantages of ATD are the possibility of remission, applicability during pregnancy and lactation, and better outcomes regarding Graves’ Orbitopathy (GO) compared to radioiodine (4). The disadvantages are the side effects, although they are usually minor and occur in 5% of the patients. Nevertheless, more rarely, life-threatening side effects can occur, such as agranulocytosis and liver damage (5). Unfortunately, 30% to 70% of patients will relapse after 12 - 18 months of ATD (4).
Thyroid-stimulating antibodies are also called long-acting thyroid stimulators, and they were found by Adams and Purves in 1956 (6). Since then, more sensitive assays have been described (7). In 1980, liquid-phase TRAb assays were utilized with detergent-solubilized TSH receptors. In the late 1990s, solid-phase TRAb assays were developed. The third generation of TRAb assays was introduced in 2003 using a human thyroid-stimulating monoclonal antibody M22, which had better sensitivity and specificity (99% for both) (8).
Thyroid-stimulating antibodies can be used in GD for many objectives, such as diagnosis (4), as well as during follow-ups to predict which patients will relapse after stopping ATD (4, 9-14). They also have important values in assessing the risks of developing GO (12) and fetal/neonatal hyperthyroidism (15, 16). This review focuses on the use of ATD and the outcomes regarding remission and TRAb development during ATD treatment.
2. Evidence Acquisition
A review was performed in Medline on the published work regarding the duration of ATD treatment and remission, ATD treatment, and TRAb development during the 1990 - 2019 period. Relevant studies would be clinical trials, cohorts, or control studies that were identified by searching Medline using the search terms “Graves’ disease and antithyroid drugs,” “Graves’ disease and long-term therapy,” “Graves’ disease and remission,” “thyroid receptor antibody/TRAb and antithyroid drugs,” “thyroid receptor antibody/TRAb and Graves’ disease,” and “thyroid receptor antibody/TRAb and remission.” We focused our analyses on clinical studies analyzing the late versions of TRAb, whenever possible. The search was done only for articles in English. All articles were reviewed independently by two reviewers (D.V. and R.B.S.).
3. Results
3.1. Serum TRAb During ATD Therapy
Graves’ disease appears to be different from other autoimmune diseases in some aspects. First, one antibody (TRAb) is primarily responsible for the disease, and second, ATD has been the only clinical treatment, which acts by restoring the euthyroidism blocking the synthesis of thyroid hormones (5). There is debate on whether the effects of ATDs on the autoimmune system are direct or indirect when controlling hyperthyroidism. The use of ATD is accompanied by a series of markers demonstrating immune system restoration, such as decreased HLA in thyroid cells (17), and decreased markers of inflammation, such as Interleucines 1, 5, and 6 and tumor necrosis factor-alpha (18, 19). There are also increases in T cell suppressors, decreases in T helper cells (20), and decreases in TRAb values over time (9, 10, 21-24).
One of the first meta-analyses on TRAb development was published 25 years ago. The analysis examined 10 studies (five prospective and five retrospective studies) that demonstrated a decline in TRAb titers during treatment, and patients with negative TRAb at the end of treatment had a protective effect against relapse (21). Capelli (25) also demonstrated a reduction in TRAb titers during the first six months of ATD treatment and correlated these levels with long-term remission. Thyroid-stimulating antibodies were also studied as a marker of remission at diagnosis (11), at 12 months of treatment (12), at the cessation of ATD therapy (> 18 months) (11, 13), and four weeks after the cessation of ATD (14).
Perhaps the most renowned study is from Lauberg (23), who prospectively studied TRAb development in 131 patients who were treated with ATD, radioiodine, or surgery. The ATD and surgery groups were significantly different from the radioiodine group. The ATD and surgery groups’ mean TRAb titers decreased and became negative around the first year. Approximately 60% of patients in the ATD group and 50% of patients in the surgery group were TRAb-negative at the end of the first year. One important point was that four patients did not achieve stable euthyroidism in the ATD group (total of 48 patients) and underwent surgery, and thus were excluded from the general analysis. These patients had higher values of TRAb, which continued to be positive, with no decrease over time until definitive treatments were performed. In contrast, the radioiodine group’s TRAb titers increased after ablative treatment and started to decrease gradually, but they were still positive even five years later.
These findings were confirmed by other studies (24), and these peaks were correlated with the development of GO (12, 26). TRAb remaining positive after years may cause fetal/neonatal hyperthyroidism through the passage of these antibodies to the placenta (4). More recently, it was demonstrated that the peak could be avoided using corticosteroid (intravenous or oral) (27). Recently, another study with a small number of patients (n = 66) had similar results when comparing the three treatment modalities (28).
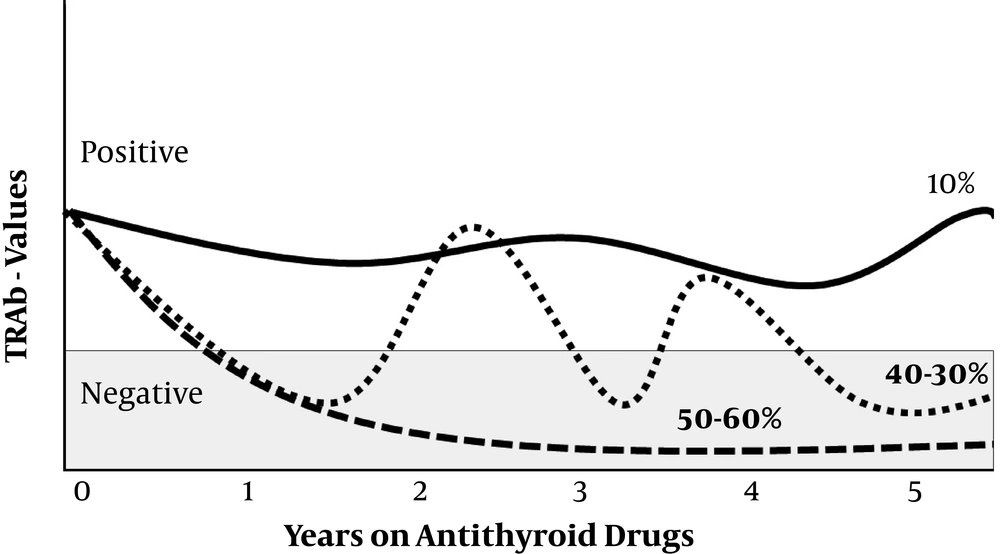
Thyroid-stimulating antibodies were also evaluated using first- and second-generation assays in 549 Japanese patients who were treated with ATD during a long follow-up period (8 - 36 years) (29). Ninety percent of patients were positive at the initial visit. These patients were divided into groups according to TRAb development: group 1: Decreasing pattern and remaining negative (40%); group 2: Fluctuating pattern (periods fluctuating between positive and negative, 40%); and group 3: Positive pattern during the first five years on ATDs (20%; about 65% of these patients became TRAb-negative at the end of the follow-up period). At the end of the follow-up period, 10% of patients still had positive TRAb titers. Group 1 had the most patients in remission (90% versus 37% in group 2 with a fluctuating pattern and 20% in group 3). Figure 1 summarizes the TRAb trend during ATD treatment.
3.2. Prolonged Treatment (More Than 18 Months) and Remission Rates
The treatment period was also the subject of several studies that analyzed the minimum and maximum intervals, influencing the remission of GD. Allannic (30) performed a prospective randomized study and observed that the remission rate of GD after two years of withdrawal from ATD was 61.8% among those treated for 18 months versus 41.7% among those treated for six months (P < 0.05). These results suggest that a longer treatment period (18 months) was more effective for the remission rate than a shorter period (six months).
A prospective trial evaluated treatment periods of 12 and 24 months. Relapse rates were 46.4% (n = 13) in the 12-month group and 54.1% (n = 13) in the 24-month group (P = ns) after two years of carbimazole withdrawal. Five years after treatment termination, the relapse rate was 85.7% in the 12-month group and 83.3% in the 24-month group (P = ns). This suggests that there would be no advantages for ATD treatment for periods longer than 24 months in terms of remission rates (31). Long periods (42 months) were also compared to 18 months, and the relapse rates were 36% and 29%, respectively (P = ns) (32).
A systematic review of the literature also evaluated treatment periods and remission rates with four trials. The authors concluded that 12 months was superior to six months of treatment, but there was no benefit in extending treatment beyond 18 months in terms of remissions rates (33). A more recent meta-analysis included six studies evaluating long periods of treatment, and there was a correlation between treatment duration and remission (16% for each year of treatment). However, it should be noted that the number of participants was too small to make a definitive conclusion. Importantly, there was a negative effect of smoking on remission rates (34) and the safe use of ATD treatment for a long period (35, 36).
This review was limited by several factors, including the difference in laboratory measurement techniques of TSH, free T4, and TRAb; the small number of patients in some studies, and the variable period of treatment.
4. Conclusions
Thyroid-stimulating antibody values usually decline during ATD treatment, but the trend could occur in two ways: becoming negative or showing a fluctuating pattern. With the use of ATD, approximately 10% of patients will remain TRAb-positive after five years of treatment. Antithyroid Drug treatment durations appear to increase remission rates, but the literature is scarce.
These findings have some clinical implications, and more research is needed. Studies should examine how we can recognize patients in which TRAb will be persistently positive. Such patients ultimately have a high risk for relapse if ATD discontinues, and perhaps, definitive treatment should be suggested or low-dose ATD should continue (34, 36). Second, research is needed to examine what events contribute to the fluctuating pattern, such as smoking or stressful events. Other issues include the meaning of increases in TRAb in the fluctuating pattern regarding GO and the ideal duration of treatment for maximal remission rates.