1. Introduction
Inappropriately high synthesis and secretion of hormones by the thyroid gland causes hyperthyroidism. This disease has multiple etiologies, presentations, and manifestations and requires various management strategies. Graves’ hyperthyroidism is an autoimmune disease caused by the stimulation of thyrotropin (TSH) receptor via TSH receptor antibodies (TRAb), resulting in the increased thyroid hormone production and release (1). The management of Graves’ hyperthyroidism involves complex decision making on diagnosis, treatment strategy, and proper follow-up (2).
Radioactive iodine (RAI) treatment and antithyroid drug (ATD) administration were introduced in the mid-1940s for the management of hyperthyroidism (3, 4). None of the therapeutic approaches for the treatment of Graves’ disease could establish normal thyroid function in the majority of patients permanently. Radioactive iodine therapy and thyroidectomy will cause lifelong hypothyroidism, and hyperthyroidism recurs in almost half of the patients following the discontinuation of conventional 12-18-month ATD treatment (2).
In the last half-century, in many countries of the world, ATDs were the first choices of the therapeutic approach for Graves’ disease, while physicians in the United States preferred RAI treatment for this purpose (5, 6). This attitude has been changed since 2005 and the majority of hyperthyroid patients in the United States are treated with ATDs in recent years (7). Because of serious liver failure complicating propylthiouracil, methimazole (MMI) is preferable for the treatment of hyperthyroidism (2). With MMI becoming the first choice in the management of Graves’ hyperthyroidism, a considerable rate of relapse after withdrawal of treatment has become a major issue. Can we predict the recurrence of hyperthyroidism, and more importantly, could we prevent its relapse?
1.1. Long-term Antithyroid Drug Therapy
Long-term ATD (LT-ATD) treatment has been proposed as an effective and safe alternative for ablative therapies of Graves’ disease (8-12). As shown in Table 1, LT-ATD treatment is favored for the treatment of Graves’ hyperthyroidism to RAI treatment, because it provides more sustained euthyroidism (8-12) and less chance of variations in serum TSH, causing lower occurrences of both subclinical and clinical hypo- and hyperthyroidism during long years of management (8, 10-17). In addition, RAI therapy is strongly associated with the worsening of thyroid orbitopathy (11, 18-20), increased BMI, and derangement of lipid profile (21-25). Furthermore, the quality of life is decreased in RAI versus LT-ATD-treated subjects (8, 26). It has also been shown that adverse events related to ATD treatment occur quite early during therapy, and occurrences of complications are rare with long-term low maintenance doses of ATD (27). The overall cost of LT-ATD treatment does not exceed (8) or may be lower than that of RAI therapy (10).
| Variables | Long-Term Antithyroid Drug | Radioactive Iodine |
|---|---|---|
| Sustained euthyroidism | More (8-12) | |
| Abnormal TSH | More (8, 10, 11, 13-16) | |
| Recurrence of hyperthyroidism | More (12, 17) | |
| Worsening of thyroid eye disease | More (11, 18-20) | |
| Increased body mass index | More (21-25) | |
| Lipid profile derangement | More (21-23) | |
| Worse quality of life | More (8, 26) | |
| Overall cost | Equal or more (8, 10) |
[object Object]
2. Arguments
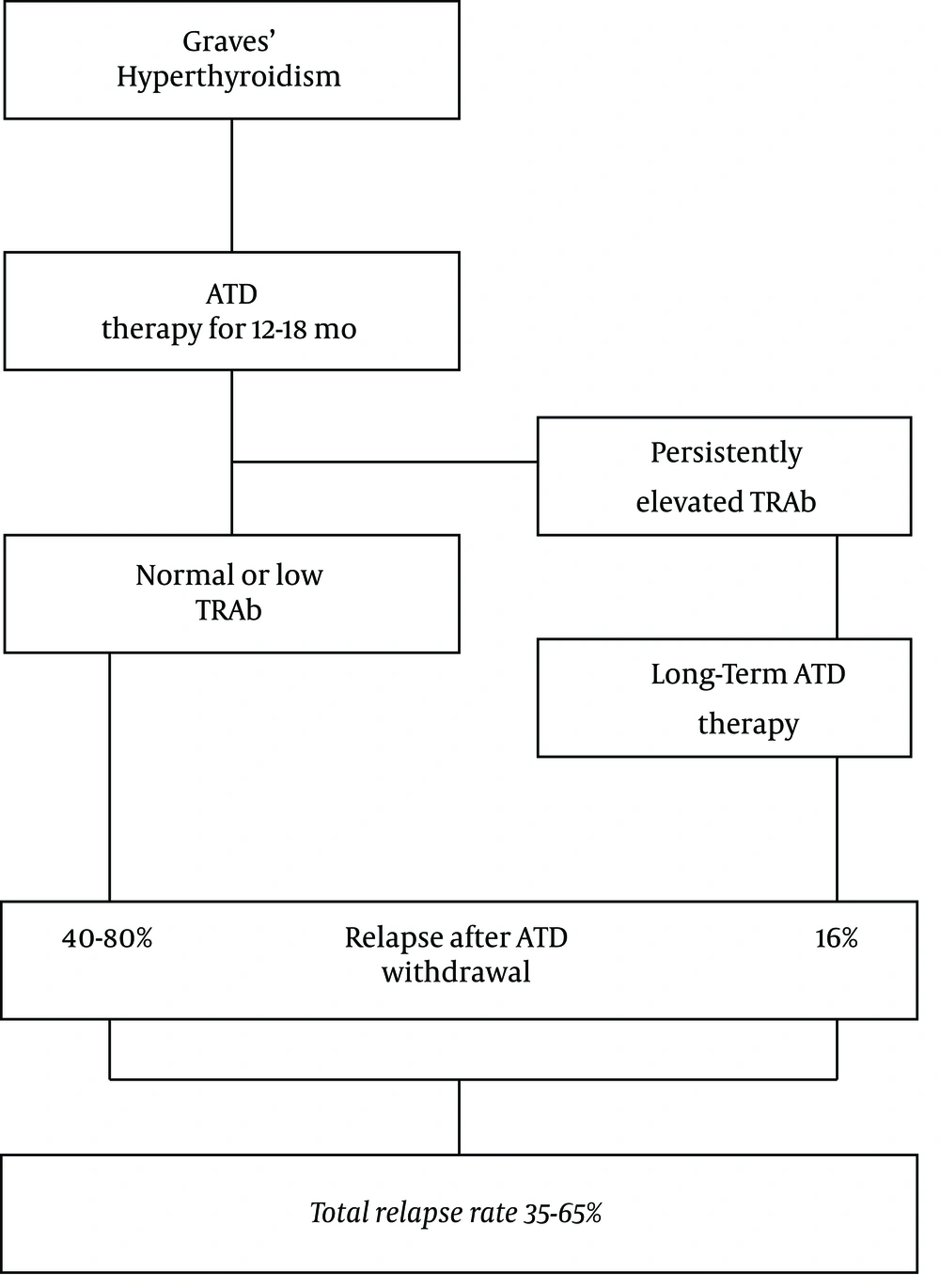
It is well known that following a conventional ATD treatment for 12 - 18 months, hyperthyroidism recurs in 20% - 70% of patients after the withdrawal of ATD (2). Many studies have tried to identify risk factors for the relapse of Graves’ hyperthyroidism. Factors identified to have stronger associations with relapse are thyroid volume, smoking, orbitopathy, more biochemically severe disease with high serum concentrations of thyroid hormones, postpartum period, prolonged treatment with ATD, and TSH receptor antibody (TRAb) titer. The latter factor has shown the strongest association although none of these factors per se is sufficient to predict the relapse of hyperthyroidism and even negative TRAb may not exclude the possibility of relapse (28). Since it was reported that patients with persistent high TRAb during ATD therapy may have an 80% - 100% chance of recurrence of hyperthyroidism (29), the 2016 American Thyroid Association (ATA) guidelines state that “continued low-dose MMI for longer than 12 - 18 months may be considered in patients, not in remission who prefer this approach”. The rate of persistently elevated TRAb (> 10 IU/L) is around 24% with an 80% - 100% chance of relapse. In addition, 64% of Graves’ patients have TRAb titers of 1.5 - 10 IU/L at the end of 12 - 18 months of ATD treatment with a 40% - 80% chance of recurrence of hyperthyroidism (30). Therefore, even if all those with persistently elevated TRAb decide to continue long-term MMI therapy, the overall relapse rate may reach 35 to 65% (Figure 1).
The relapse rate of hyperthyroidism following discontinuation of ATD therapy according to 2016 ATD guidelines (2). After 12 - 18 months of ATD therapy, 24% of patients would have persistently elevated TRAb with at least a 16% chance of relapse if they continued long-term (≥ 60 months) ATD therapy. 64% of patients would have TRAb levels of 1.5 - 10 IU/L with a 40% - 80% chance of relapse. Altogether, more than 35% - 65% of patients would show the relapse of hyperthyroidism (Adopted from references 2 and 12). Abbreviations: ATD, antithyroid drug; TRAb, thyrotropin receptor antibody.
In original studies after the discovery of ATD, it was believed that increasing the duration of ATD therapy would not induce any further decreases in the relapse rate following withdrawal (31) and guidelines advised 12 - 18 months as the optimal duration for effective ATD treatment (1, 2). Recently, a few studies have reported that a longer duration of ATD therapy can cause a higher remission rate and a meta-analysis showed that indeed this is true, and each one year of additional ATD treatment may add 16% to the remission rate of conventional therapy (32). This finding is very important because, among all risk factors showing strong associations with relapse, only the duration of ATD therapy is a changeable factor. In addition, studies have shown that ≥ 60 months of continuous MMI treatment is accompanied by a very high remission rate of 84% up to four years after the discontinuation of ATD therapy, in both adults (12) and children (33).
In the last decade, attempts have also been made to use a combination of risk factors for the prediction of relapse of Graves’ disease. Vos et al. (34) reported a risk score based on age, large goiter, higher fT4, and higher TBII. The relapse rates in class I, II, and III of this so-called Graves’ recurrent events after therapy (GREAT) score were 16%, 44%, and 68%, respectively. The GREAT scoring system has been validated in three studies (35-37). Table 2 shows the results of GREAT risk scores in four studies. The recurrence of Graves’ disease has occurred in 12% - 34% of class I, 35% - 59% of class II, and 53% - 74% of class III patients with Graves’ disease.
aGREAT scoring, first reported by Vos et al. (34), includes age ≥ 40 (0) and < 40 years (1+), goiter size 0 – 1 (0) and 2 - 3 (2+), serum free thyroxine < 40 (0) and ≥ 40 pmol/L (1+), serum thyrotropin binding inhibitory immunoglobulin (TBII) < 6 (0), 6 - 19.9 (1+) and ≥ 20 (2+); the maximum score is 6; risk stratification: 0 - 1 (class I), 2 - 3 (class II), and 4 - 6 (class III).
3. Conclusions
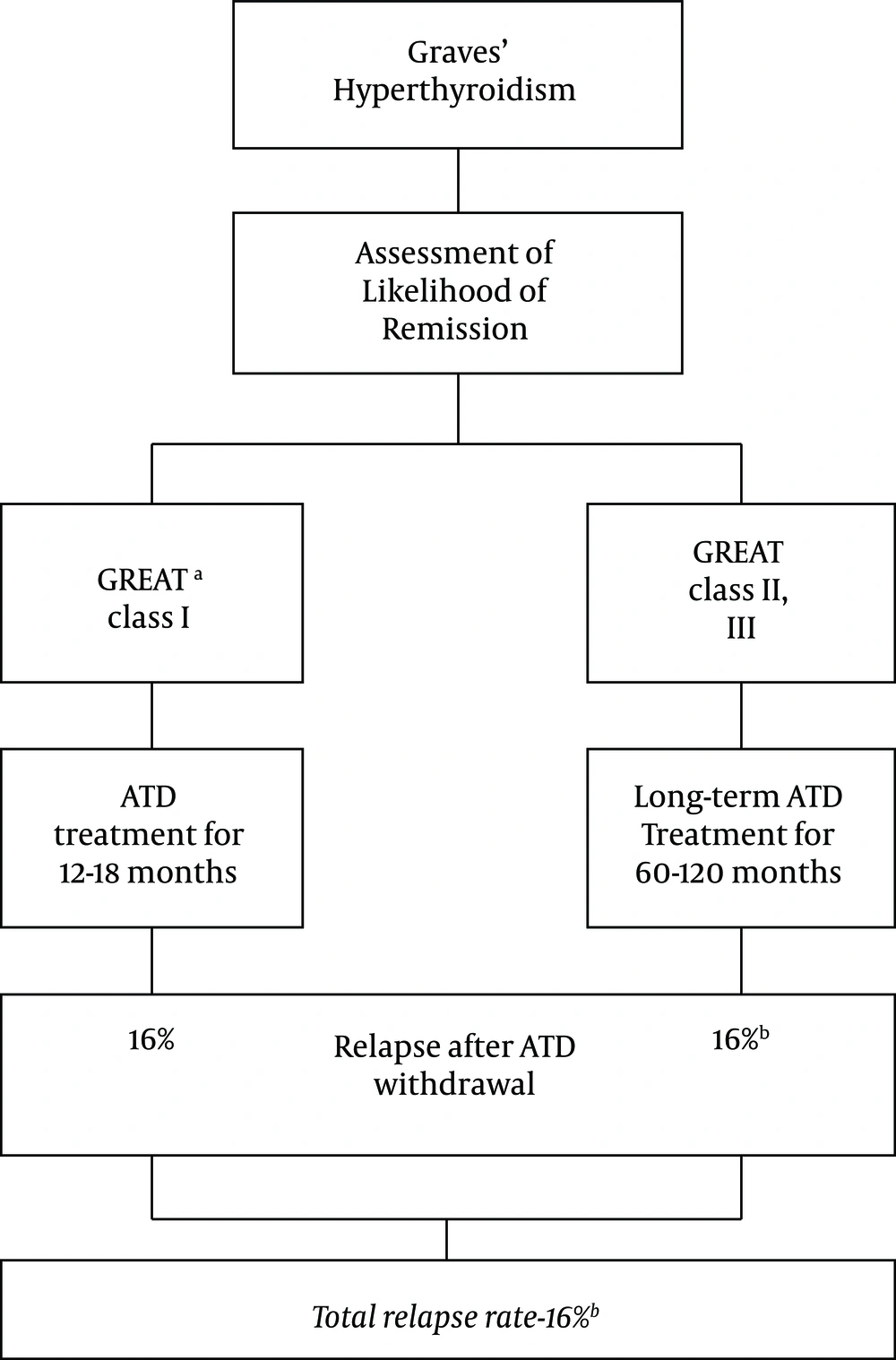
Taking all recent studies mentioned above, a new approach may be proposed for the management of Graves’ disease. According to this approach, before the start of ATD treatment, one could separate those with class I GREAT scores and treat them with 12 - 18 months of MMI; the relapse rate in this group of patients is 16% (34). However, those with class II and III GREAT scores would be treated with ≥ 60 months of MMI treatment. The relapse rate in this group of patients with more severe hyperthyroidism after long-term continuous MMI treatment would be estimated at 16% or slightly higher (12). Therefore, the proposed approach may cause a cure in more than three-fourth of patients with Graves’ hyperthyroidism (Figure 2). The superiority of this approach to ATA 2016 guidelines awaits well-planned clinical trials. In addition, it would be helpful to include the information on TRAb changes during treatment to decide on ATD withdraw (38, 39).
The relapse rate of hyperthyroidism after ATD withdrawal according to a new concept. 16% of Graves’ patients would have mild (GREAT class 1) disease and 16% of them would experience relapse after 12 - 18 months of ATD therapy (34). Remaining patients (GREAT class II and III) would be treated with long term (≥ 60 months) ATD and would have a 16% or slightly higher relapse rate after ATD withdrawal (12). Altogether, more than three-fourth of patients would experience remission of hyperthyroidism. a: Graves’ recurrent events after therapy score (34); b: May be slightly higher.
It is noteworthy to consider that LT-ATD therapy has become an option for the control of hyperthyroidism. This mode of treatment may not apply to all patients with Graves’ disease, since some patients may have adverse events or choose RAI or thyroidectomy. In addition, the patient’s choice to carry on with long-term ATD therapy will be an important deciding factor.
It is concluded that guidelines for the management of hyperthyroidism should take into consideration the new evidence for the prediction and prevention of Graves’ hyperthyroidism. Long-term ATD treatment is both effective and safe and can cause more cure than other modes of treatment for Graves’ disease (40). Adverse events of therapy occur mostly in the first few months of therapy and they are extremely rare during low maintenance dose of continuous long-term ATD treatment (16). Therefore, physicians and patients may consider this mode of therapy for their decisions on medical versus ablative therapies for Graves’ hyperthyroidism.