1. Context
Graves’ disease (GD) is the most common cause of hyperthyroidism, which is caused by a complex relationship between genetic factors and environmental influences. Essentially, the disease is caused by the appearance of stimulating TSH receptor autoantibodies (TRAb), leading to uncontrolled hyperfunction of the thyroid gland. The majority of patients have a prolonged course with remission and relapse over many years (1).
Management of GD includes antithyroid drugs (ATD), radioiodine (RAI) therapy, and thyroidectomy. ATD is the first-line treatment, which is continued for a period of 12 to 18 months. A survey conducted in 2011 from clinically active international members of The Endocrine Society, the American Thyroid Association, and the American Association of Clinical Endocrinologists reported that the preferred mode of therapy in uncomplicated GD was ATD (53.9%), followed by RAI (45.0%) and thyroid surgery (0.7%) (2). A more recent study conducted in 2016 in the United States of America revealed a change in the preferred treatment method from RAI (35%) to ATD (58%) (3); while in 1991, 69% of physicians in North America recommend RAI as a first-line therapy (4). Over the last few decades, ATD has been recognized worldwide as the first choice for the treatment of hyperthyroidism.
The primary goal of ATD therapy is to temporarily restore the patient to a euthyroid state. At the same time, we anticipate disease remission and reduction in iatrogenic hypothyroidism, which is often seen in thyroidectomy or RAI radiation. Furthermore, RAI is associated with a definite risk for worsening or developing of Graves’ ophthalmopathy.
Remission from GD is defined as the maintenance of biochemical euthyroidism for at least 1 year following the discontinuation of ATDs (5). Relapse following treatment occurs approximately 30% - 40% in the first year and 50% - 60% in the long term (6-11). An autoimmune disease should theoretically be viewed as a recurring disease. Hence, the length of remission may be a better method to evaluate treatment response. This method is superior to simply categorizing patients into treatment success and failure groups. Furthermore, the switching of hyperthyroidism to hypothyroidism or alternating hyperthyroidism and hypothyroidism has been reported in the follow-up of patients with GD (12). An analysis of the long-term outcome of patients in stable remission, for more than 10 years, revealed euthyroidism in 60%, subclinical hyperthyroidism in 16%, subclinical hypothyroidism in 18%, and hypothyroidism in 6% (13-15).
Therefore, we need useful parameters to guide clinicians in finding out which patients are suitable candidates for ATD treatment.
2. Evidence Acquisition
PubMed was used to search English-language literature from 1995 through to 2019, using the following search terms: Graves’ disease, hyperthyroidism, antithyroid drugs, outcome, relapse, and recurrence. Reference lists from review articles and textbooks were included in the search in order to find older papers. Systemic review, meta-analysis, case series, retrospective, and prospective cohort studies were also included. This review is mainly focused on studies concerning adult GD, with patients aged < 18 years of age being excluded. We primarily focused on this population because adolescents and children generally have higher rates of relapse (16, 17).
3. Results
Factors associated with a low remission rate, as reported in most studies, can be divided into phenotype and genotype predictors.
3.1. Phenotype Predictors
3.1.1. Goiter size and Severe Thyrotoxicosis
Goiter size reflects the response of the functional mass to TSH receptor stimulation. Nearly all reports have confirmed a strong relationship between the goiter size and chance of relapse (8, 18-24). Baseline T4 and T3 values also have predictive value. Higher levels of T4 and T3 are associated with a greater chance of relapse (18, 19). Additionally, there is a correlation between goiter size and biochemical hyperthyroidism (19, 21).
3.1.2. Persistent Positive TSH Receptor Antibody
Either a high TRAb at diagnosis or a positive TRAb at the end of therapy or both were associated with a high likelihood of relapse (18, 20, 22, 23, 25-28). Negative TRAb is a good predictor for remission, and persistently high TRAb (> 10 IU/L) during ATD therapy is associated with an 80% - 100% chance of hyperthyroidism recurrence (29). However, 64% of Graves’ patients have TRAb titers of 1.5 - 10 IU/L at the end of the 12 - 18 months of ATD therapy, and 40% - 80% may have a recurrence of hyperthyroidism after discontinuing ATD therapy (30). We found that baseline TRAb did not predict relapse in some studies (7, 27), whereas a persistently high TRAb titer after therapy was a more predictive value (7, 28). A recent study conducted in Japan reported long-term outcomes of ATD treatment in 549 adult GD patients, including a follow-up period of more than 8 years (31). This study found that baseline a degree of thyroid dysfunction, thyroid volume, or TRAb levels were not predictive; however, there was a correlation between the patterns of the disappearance of serum TRAb concentrations over time and disease remission (31). Patients with fluctuating TRAb titer had a lower rate of remission (37.2%) than those with a smooth non-fluctuating type (88.9%) at the end of the follow-up period.
3.1.3. Past History of Recurrence
The risk for relapse may be higher in patients with more than one course of drug therapy (7, 32). In a study by Wang et al. (7), patients with a second occurrence had a higher rate of relapse than those presenting with an initial occurrence (84% vs. 43%). Notably, nearly all of the patients with two or more previous relapses had a recurrence after drug withdrawal. Schleussner et al. (22) reported that the rate of relapse in patients with an initial occurrence (47%) was similar to those with one relapse (50%), while patients with more than one prior relapse had a 75% relapse rate. A Korean study demonstrated that the 10-year remission rates were 34%, 25%, 17%, and 13%, respectively, in those with a first, second, third, and fourth course of ATD treatment (32).
3.1.4. Smoking
Smoking is a risk factor for the development of clinically overt thyroid disease (33), GD relapse (18, 20), and the development of more severe ophthalmopathy (34, 35). Nyirenda et al. (36) found that compared to nonsmokers, smokers showed a much slower reduction in concentrations of TRAb, FT4, and T3 under carbimazole treatment. Kimball et al. (37) found that the effect of smoking was highly significant in males, and thus concluded that cigarette smoking increased the likelihood of GD recurrence in males treated with antithyroid drugs. Two studies confirmed previous findings on recurrence after drug withdrawal in Taiwanese (23, 38) and Belgian (39) populations. A recent systematic review and meta-analysis by Azizi et al. (16) revealed that smoking had a significant effect on lowering remission rates. Another recent study conducted in Sweden, reported a very low relapse rate in patients who had previously quit smoking (8). This study deserves further investigation and may encourage GD patients to abstain from smoking after ATD withdrawal.
3.1.5. Graves’ Ophthalmopathy
A systemic review and meta-analysis by Struja T et al. (18) revealed that orbitopathy was significantly associated with relapse. In a retrospective observational study of 158 patients with a well-defined assessment of Graves’ ophthalmopathy (GO), 27 out of 65 patients (42%) with mild GO experienced remission during ATD treatment; whereas only 7 out of 93 patients (8%) with severe GO achieved remission (40). The degree of GO was included as a parameter to evaluate the clinical severity score (CSS) and has been linked with poor hyperthyroidism control after 6 months of ATD treatment (41). Numerous other studies have not found any correlations between the presence of GO and the outcome after ATD treatment (8, 19, 27, 42).
3.1.6. Age and Sex
Younger patients have been considered to have higher rates of relapse (17, 22, 43), although a recent systemic review and meta-analysis revealed no significant association (18). Male sex was linked with a higher relapse rate in some studies (27, 43); however, this has not been a universal finding (18, 22).
3.1.7. Duration of Therapy
Both retrospective and prospective analyses have suggested that a longer duration of drug therapy is associated with higher remission rates (44, 45). A systematic review and meta-analysis of long-term (> 24 months) ATD treatment conducted by Azizi et al. (16) suggested a positive relationship between the length of treatment and remission rate, although it was not a true linear correlation. Conversely, other researchers have suggested that treatment duration is of little importance (7, 46). The consensus may be that there is a high recurrence rate after only 6 months of treatment, and no additional benefits gained by extending therapy beyond 18 months (46). A study by Wang et al. (7) showed that long-term (≥ 18 months) therapy was associated with a higher relapse rate. This was likely related to the clinical selection of patients with larger goiter and recurrence in the past to receive long-term therapy (7).
3.1.8. Combined Antithyroid Drug-Thyroxine Therapy
Theoretically, treatment with thyroid hormone should decrease serum thyrotropin levels and lower the expression of certain thyroidal antigens, which then may stop the perpetuation of the autoimmune response (47). The block-and -replace method may smooth out the clinical course, but the remission rates do not differ between the titration method and block-and-replace method in most studies (7, 48, 49).
To the best of our knowledge, the phenotype factors particularly bound to subsequent relapse are large thyroid volume, smoking, and the persistence of TRAb in the circulation at the end of treatment (20, 23).
3.2. Genotype Predictors
Genetic factors have been known to play a role in this condition. Specifically, the interaction between major histocompatibility complex (MHC) genes (22, 42, 50-55) and non-MHC genes (i.e., immunoregulatory genes and specific autoantigen genes). The immunoregulatory genes that affect GD development are the cytotoxic-T lymphocyte-associated protein 4 (CTLA-4), CD40 (56-59), and protein- tyrosine phosphatase-22 (PTPN22) (60, 61). The thyroid-specific genes are thyroglobulin (38) and thyroid-stimulating hormone receptor (62, 63).
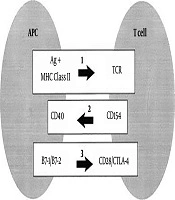
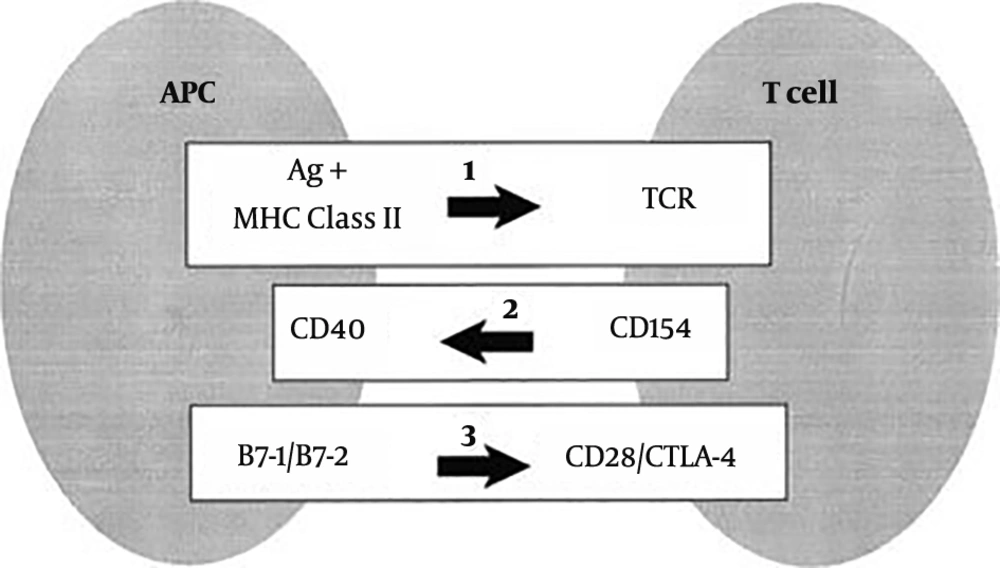
Graves’ disease requires two signals in order to activate the T cells: (1) Specific binding of the T-cell receptor (TCR) to the peptide antigen bound to the HLA class II molecules on antigen-presenting cells; and (2) antigen-independent costimulatory pathway to generate subsequent cytokines and cell surface molecules. TCR engagement alone is not sufficient for the complete activation of T cells. The costimulatory pathway is a highly organized multi-step program, including afferent and efferent signals. In the afferent pathway, the importance of the co-receptors CD28, inducible costimulatory molecule (ICOS), and cytotoxic T lymphocyte antigen 4 (CTLA4) is recognized. CD28 and ICOS have both overlapping and distinct functions in the positive regulation of T cell responses, whereas CTLA4 negatively regulates the response (56, 57). In the efferent pathway, CD40L (CD154) is expressed briefly on CD4 T cells after they are activated through the TCR. CD40L promotes the formation of memory B cells and contributes to B cell proliferation. CD40L is essential for the crosstalk of T cells with B cells (58, 59) (Figure 1).
Major histocompatibility complex (MHC) and immunoregulatory genes. 1, Stimulation of the T-cell receptor (TCR) by recognition of antigen (Ag) in the context of class II major histocompatibility complex (MHC) molecules results in the expression of CD154 on T cells. CD154 (CD40L) binds to CD40 on antigen-presenting cells (APCs) and enhances B7-1 and B7-2 expression. B7:CD28 interactions also promote CD154 expression. CTLA-4 binding to the B7 molecules blocks CD28 activation by B7. CD28 and CTLA-4 represent the “general switches” and strongly influence the expression of many downstream costimulators (64).
3.2.1. Major Histocompatibility Complex
The HLA complex is located on chromosome 6 and contains sequences encoding the genes that are involved in the regulation of the immune response (50). HLA Class II haplotypes DRB1-03, DQA1-05, and DQB1-02 are well documented as being associated with susceptibility for Graves’ hyperthyroidism development (51-53). Although controversy still exists (22, 54, 55), Vos et al. (42) found that HLA DRB1-03, DQA1-05, and DQB1-02 polymorphisms are strong predictors for recurrence after ATD therapy.
3.2.2. The Costimulatory Genes
The costimulatory genes, which are not involved in the initiation of autoimmune response to thyroid antigens, provide a critical second signal for naive T cell activation after the initial binding of TCR to the antigenic peptide-MHC complex.
Focusing on the relapse of GD, costimulatory genes play a more crucial role than the genes responsible for disease susceptibility such as HLA (50-52) or TSHR (62, 63). A study by Wang et al. confirmed the associations between costimulatory genes and relapse of GD after ATD cessation (23, 65). SNP exon 1 + 49 A/G (rs231775) of the CTLA-4 gene in the afferent signal of costimulatory molecules, and three other risk alleles (rs745307, rs11569309, and rs3765457) at the CD40 in the efferent signal, were found to be risk alleles for recurrence. Grouping risk alleles in afferent signal (CTLA-4) and risk alleles in efferent signal (CD40) improved the predictability of relapse (23). In their subgroup analysis by age, harboring risk alleles (genetic factors) were found to be better predictors of recurrence in the younger group, while goiter size (clinical sign) was more useful in the older group (23).
A study conducted in Japan also found that remission of GD was associated with the A/G polymorphism at position 49 in exon 1 of CTLA4 by analyzing the time of TRAb disappearance after the start of ATD treatment (66). More recently, the CTLA-4 A/G genotype has been demonstrated as an independent predictor of long-term remission following a first course of ATD in a Spanish population (67). However, Kim et al. (68) did not find an association between GD relapse and the risk alleles of CTLA-4 and CD40 genes. In their study, they used a simple dichotomous classification method where patients were grouped into remission and failure groups with a cutoff point of 12 months after withdrawal of ATD treatment; this classification system could have contributed to the lack of correlation indicated by their data set.
3.2.3. Protein Tyrosine Phosphatase-22 Gene
The PTPN22 gene on chromosome 1p13.3-13.1 encoding for protein tyrosine: Phosphatase-22 is a powerful inhibitor of T-cell activation. The common 1858T (rs2476601) Arg620Trp nonsynonymous single nucleotide polymorphism located in the PTPN22 gene has been associated with the development of multiple autoimmune diseases, including type 1 diabetes mellitus, rheumatoid arthritis, systemic lupus erythematosus, as well as autoimmune thyroid diseases (60, 61). A correlation between PTPN22 gene polymorphisms and a susceptibility to Graves’ disease has been detected in Japanese (69), Polish (70), Russian (71), Mexican (72), and British Caucasian (73) populations. Vos XG et al. were the first to find that PTPN22 C/T SNP (rs2476601) is also associated with the recurrence rate (42). Nevertheless, more studies are required to provide further confirmation.
3.3. Multi-Marker Prediction Models
The sensitivity and specificity value of each of these risk factors was too low to be useful for daily clinical decisions in the treatment of an individual patient. Therefore, multi-marker prediction models have been proposed in order to improve the predictive value of clinical and genetic parameters. In 2016, Vos et al. (42) constructed The Graves’ recurrent events after therapy (GREAT) score for clinical markers; the GREAT+ score was also developed for combining clinical and genetic markers in order to calculate the risk of recurrence prior to the start of ATD treatment. The data was derived from 178 patients with a first episode of GD who were treated with ATD (block and replacement regimen) for 1 year, and were followed for a further 2 years. The GREAT and GREAT+ scores were divided into classes according to baseline recurrence risk factors (i.e., age, serum fT4, serum TBII, goiter size, HLA polymorphism, PTPN22 C/T). Table 1 shows the recurrence rates at the end of the follow-up in GREAT score classes I, II, and III which were 16.4%, 43.9%, and 68.4%, respectively. The recurrence rates at the end of follow-up in GREAT+ score class I, II, III, and IV were 4.3%, 20.6%, 49.3%, and 84.2%, respectively. The largest benefit of the GREAT+ score is in GREAT score class II. In 2017, Bartalena et al. (41) developed a Clinical Severity score (CSS) to assess the overall disease severity by grading each component of the Merseburg triad (i.e., hyperthyroidism, goiter, and orbitopathy). The baseline CSS score was associated with a poor control of hyperthyroidism after 6 months of ATD treatment and a non statistically significant risk for hyperthyroidism at month 12. However, neither goiter size nor serum fT4 levels were per se associated with a risk of poor control (41).
| Marker | Great Score | Great + Score |
|---|---|---|
| Age, y | ||
| ≥ 40 | 0 | 0 |
| < 40 | +1 | +1 |
| Serum fT4, pmol/L | ||
| < 40 | 0 | 0 |
| ≥ 40 | +1 | +1 |
| Serum TBII, IU/L | ||
| < 6 | 0 | 0 |
| 6 - 19.9 | +1 | +1 |
| ≥ 20 | +2 | +2 |
| Goiter sizea | ||
| 0 - I | 0 | 0 |
| II - III | +2 | +2 |
| HLA polymorphisms, nb | ||
| 0 | 0 | |
| 1 - 2 | +2 | |
| 3(LD) | +3 | |
| PTPN22 | ||
| Wild type | 0 | |
| C/T | +1 | |
| Maximum score | 6 | 10 |
| Risk stratification | 0 - 1 (class I) | 0 - 2 (class I+) |
| 2 - 3 (class II) | 3 - 4 (class II+) | |
| 4 - 6 (class III) | 5 - 6 (class III+) | |
| 7 - 10 (class IV+) |
Coefficients for the GREAT and GREAT+ Scores: Predictive Scores Based on Baseline Characteristics for Recurrence (39)
The predictive value of the GREAT score has since been validated by a study retrospectively analyzing the data of 741 patients with a first episode of Graves’ hyperthyroidism from four Swiss endocrine outpatient clinics (74); in addition to a retrospective single-center study of 387 GD patients, who completed an 18 - 24 months ATD course and were followed for at least 2 years in Italy (75). However, if more genetic markers, such as CTLA4 and CD40 were included, the predictive value would be enhanced.
4. Conclusions
GD is an autoimmune disease, and the majority of patients have a prolonged course with remissions and relapses over many years. The primary goal of ATD therapy is to temporarily restore the patient to the euthyroid state, and to wait until disease remission. The current consensus is that longer time for TRAb normalization, the persistence of a palpable goiter, and harboring genetic risk factors in younger patients are associated with high recurrence rate of GD. The sensitivity and specificity value of each of the phenotype or genotype risk factors is too low to be useful for daily clinical decisions in the treatment of an individual patient.