1. Background
Heart failure is a pathophysiological condition in which the heart’s output is not able to establish the body’s needs for oxygen and nutrients. Failure of the heart to discharge or fill up increases the filling pressure and end-ventricular diastolic volume, and so the affected person cannot perform his or her activities without symptoms such as shortness of breath or fatigue and weakness (1-3). The prevalence of heart failure has increased dramatically in recent decades. As many as 1% - 2% of the population in developed countries has chronic heart failure, and the rate reaches 10 percent over the age of seventy (4, 5).
Vitamin D deficiency, similar to smoking, high level of cholesterol and low-density lipoprotein (LDL), low level of high-density lipoprotein (HDL), high blood pressure, diabetes mellitus, and stress, is one of the controllable risk factors of cardiovascular diseases (6). Studies showed that the level of vitamin D in patients with chronic heart failure is lower than the healthy people of the same age (7), and vitamin D deficiency in patients with chronic heart failure has a direct relationship with mortality (8). Some of the causes of vitamin D deficiency in these patients include inactivity and getting inadequate sunlight for vitamin D synthesis, as well as nutritional deficiency (9).
Vitamin D is made when ultra-violet (UV) reacts with 7-dehydrocholesterol in the skin, and eventually, this compound is converted to its biologically active form called 1 and 25-hydroxy vitamin D in the kidneys (10, 11). Recent studies showed that in addition to the role of vitamin D in bone mineralization and intestinal calcium transmission, it has receptors in the vascular endothelium, smooth muscle, and heart (12), and its deficiency increases the risk of acute coronary syndrome by 7% (13). Also, vitamin D has been shown to be deficient in diseases such as hypertension, diabetes mellitus, and metabolic syndrome and acts as a protective factor against cardiac hypertrophy and myocardial dysfunction (14, 15).
Therefore, given the importance of chronic heart failure, morbidity, and mortality considerations and imposing heavy costs on the health system, proper diagnosis, and treatment of these patients are of great importance.
2. Objectives
This study aimed to evaluate the effect of vitamin D on the improvement of left ventricular ejection fraction in patients with systolic heart failure and whether it can be used as a complementary therapy. Also, to address the type of vitamin D intervention, whether it is a supplement, an enrichment supplement, or a dietary approach. This will cause an improvement in patients’ quality of life, increasing left ventricular function and, ultimately, better outcomes in patients with chronic heart failure.
3. Methods
This double-blind, randomized clinical trial was performed from November 2016 to October 2018 in Mousavi Hospital of Zanjan with ethical code: ZUMS.REC.1395.205 and IRCT ID: IRCT20190410043236N1. Eighty-two patients with chronic systolic heart failure, after obtaining informed consent, were divided into the two following groups: the intervention group (who received 50,000 units of vitamin D weekly for eight weeks, manufacturer: Abidipharma company) and the placebo group (who received the placebo with the same appearance, dose, and duration of the real drug; but different content, biochemical content: Vitamin D gelatinous pumice, manufacturer: Iran capsule gelatin production company). In this study, sampling of the statistical population was performed by simple and completely random methods, and concealment from patients admitted to the coronary care unit or cardiac ward. Also, the study was double-blind, and the cardiologist who performed echocardiography did not know the patient grouping, and patients did not know any of the types of substances received (placebo or vitamin D). The patients were evaluated after taking vitamin D or placebo.
In the beginning, a checklist, including demographic information of patients, comorbidities, and medications for chronic heart failure, was used. The inclusion criteria of the study were chronic heart failure patients with 20% ≤ ejection fraction (EF) ≤ 45% (according to Framingham criteria, New York Heart Association (NYHA) Functional Class I, II, III) and 25 (OH) vitamin D levels less than 30 ng/dL, and patients over 40 years of age. Patients with hypercalcemia, osteomalacia, Corticosteroid treatment, and renal failure were excluded from the study. Before drug administration, left ventricular ejection fraction (LVEF) and heart failure rating based on NYHA ratings were calculated by vivid echocardiography using Biplane Simpson method, and ventricular wall thickness was also recorded by a qualified cardiologist. To determine the LVEF, two-dimensional echocardiography was used, which has a sensitivity and specificity of 83% and 82%, respectively (16). Echocardiography was also performed at all stages by a cardiologist. The cardiologist who performed the echocardiography did not know the patient grouping. The etiology of heart failure, including hypertension, ischemic heart disease, valvular heart disease, or cardiomyopathy, was detected based on history, echocardiographic findings, and patients’ previous dossiers for each patient and was recorded in the checklist. Laboratory tests for these patients included serum levels of phosphor, creatinine, calcium, and albumin. These tests were checked immediately before and after the intervention. These tests were assessed as immunoassay with liquid chromatography-tandem mass spectrometry systems (LC-MS/MS) in the laboratory of Mousavi Hospital. In this study, ejection fraction (EF), end-diastolic volume (EDV), and end-diastolic wall thickness (EDWT) were considered the main outcomes, and serum levels of phosphor, creatinine, calcium, albumin, and vitamin D were considered the secondary outcomes.
Confounder variables in this study were: patients with different vitamin D levels, age differences, factors affecting serum vitamin D levels, including hypercalcemia, osteomalacia or corticosteroid therapy, and renal failure, as well as effective cardiac causes in exacerbating heart failure, including acute cardiovascular events. Using the inclusion and exclusion criteria, it was attempted to remove confounding variables as much as possible before and during the study.
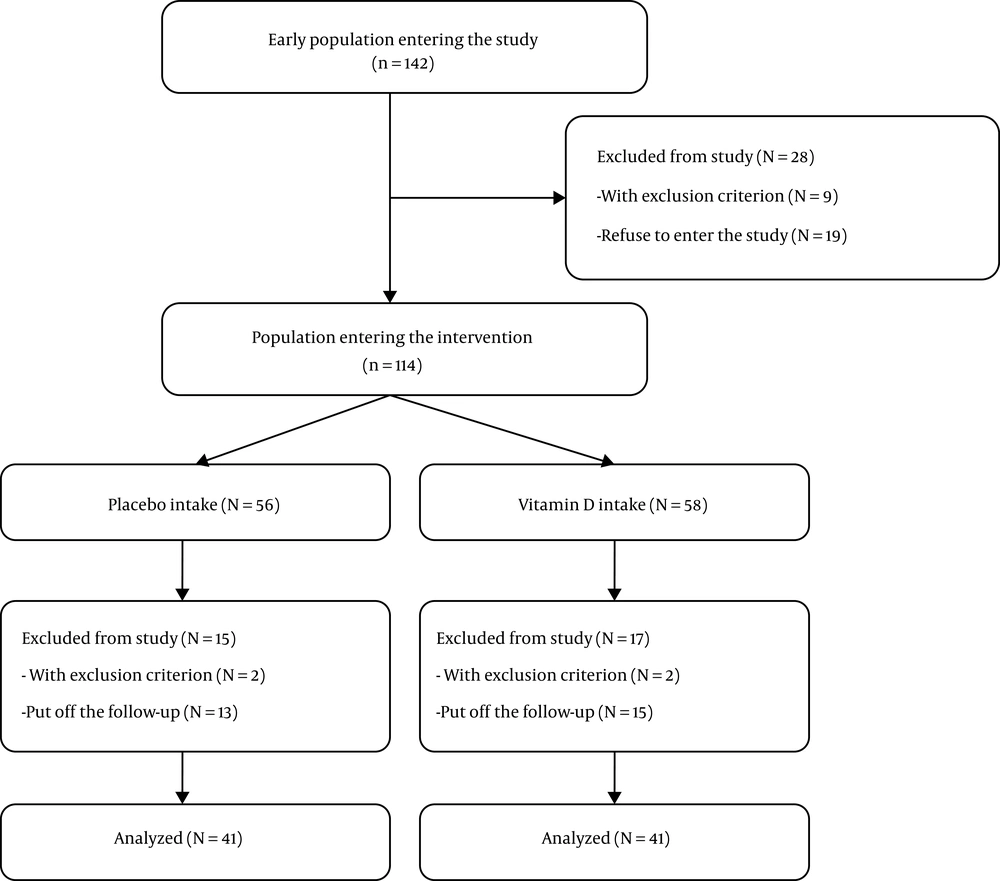
To determine the sample size, the target-based method was used, and was obtained based on previous similar studies (7, 17) and due to time constraints and geographical limitations of the number of heart failure patients. The sampling flowchart was designed according to the CONSORT guideline. In the present study, 142 patients with chronic systolic heart failure were enrolled and 28 patients were excluded (9 patients with exclusion criteria and 19 patients with refusal to enter the study). Finally, 114 patients were included and divided into two groups in the form of a computer-generated random number order. It should be noted that patients were not aware of the type of drug. The patients were followed up for 2 months. Heart failure decompensation, changing medications for heart failure, progressive kidney failure with over 2 creatinine level test, requiring revascularization due to coronary artery disease, progression to heart failure NYHA Class IV, and death were exclusion criteria of the study during the follow-up. Owing to not referring to follow-up on time and according to the exclusion criteria, 15 patients were excluded from the placebo group and 17 patients from the intervention group during the follow-up (Figure 1). To ensure regular and timely use of vitamin D and placebo, as well as to re-emphasize to py attention to the effective items in vitamin D level, the researchers contacted patients under follow-up every two weeks by telephone. Also, these patients were visited in the clinic every month. To assess patient compliance, we checked the number of non-used vitamin D and placebo tablets.
3.1. Statistical Analysis
The study was completed after 2 months with repeated vivid echocardiography and ejection fraction measurements and 41 patients in the intervention group and 41 patients in the placebo group were analyzed. Inferential statistics were first analyzed using the Kolmogorov-Smirnov test. In the next step, in case of a normal distribution of data, for the ejection fraction and laboratory testing, an independent t-test was used to compare the two groups, and in case of non-normal distribution of data, for the NYHA Class, the Mann-Whitney U-test was used. Statistical analyses were performed using SPSS software version 23 and the P value of less than 0.05 was considered significant.
4. Results
In this study, according to the inclusion and exclusion criteria, finally, 114 patients were included and divided into intervention (n = 58) and placebo (n = 56) groups in the form of a computer-generated random number order. During the study, owing to not referring to follow-up on time and according to the exclusion criteria, 15 patients from the placebo group and 17 patients from the intervention group were excluded. Eventually, ventricular ejection fraction was evaluated in 82 patients with systolic heart failure who received vitamin D as the intervention group and placebo as the placebo group.
Table 1 shows the baseline characteristics of the study population in the placebo and intervention groups. The results showed no significant difference between the two groups in terms of baseline variables. The intervention group consisted of 18 female and 23 male patients with a mean age of 61.68 ± 19.8 years. The placebo group included 21 female and 20 male patients with a mean age of 62.12 ± 18.2 years. In both the intervention and placebo groups, hypertension and coronary artery disease were the most common diseases associated with chronic heart failure. The findings also showed that the majority of patients in both groups were in NYHA Class ΙΙ (Table 1).
| Variable | Intervention Group | Placebo Group | P Value |
|---|---|---|---|
| Age | 61.68 ± 19.8 | 62.12 ± 18.2 | 0.847 |
| Sex | 0.507 | ||
| Male | 23 (56.1) | 20 (48.7) | |
| Female | 18 (43.1) | 21 (51.2) | |
| Etiology of systolic heart failure | |||
| HTN | 27 (65.8) | 29 (70.7) | 0.635 |
| Diabetes | 10 (24.3) | 9 (21.9) | 0.754 |
| Dyslipidemia | 12 (29.2) | 11 (26.8) | 0.806 |
| Coronary artery disease | 29 (70.7) | 27 (65.8) | 0.635 |
| Cardiomyopathy | 4 (9.7) | 3 (0.7) | 0.639 |
| Valvular | 8 (19.5) | 11 (26.8) | 0.432 |
| NYHA class | 0.744 | ||
| Class Ι | 5 (12.2) | 4 (9.75) | |
| Class ΙΙ | 29 (70.73) | 32 (78.04) | |
| Class ΙΙΙ | 7 (17.07) | 5 (12.2) |
>Abbreviation: NYHA class, New York Heart Association Functional Classification.
aValues are expressed as No. (%) or mean ± SD.
Table 2 shows the variables studied before the intervention and placebo as well as the primary and secondary outcomes of the study in the two groups after the intervention. Evaluations showed that there were no significant differences between the study variables of the two groups before the intervention. After two months of the intervention with vitamin D and placebo, the main outcomes of this study, including changes in ejection fraction and end-diastolic volume, were significantly different between the two groups. Comparison of the secondary outcomes of the two groups showed that the serum level of albumin and vitamin D in the intervention group was significantly higher than the placebo group. However, there was no significant difference in serum levels of creatinine, calcium, and phosphor between the two groups (Table 2). Also, the improvement of heart failure class after two months of the intervention was significantly different between the two groups such that the improvement of NYHA class to another NYHA class was seen more in the intervention group than the placebo group (Table 3).
| Variable | Intervention Group, | Placebo Group, | P Value | P Value (Based on the Amount of Changes) |
|---|---|---|---|---|
| EF, % | < 0.001 | |||
| Before the intervention | 29.93 (6.72) | 31.05 (7.05) | 0.463 | |
| After the intervention | 35.39 (6.15) | 31.41 (6.83) | 0.007 | |
| EDV, mL | < 0.001 | |||
| Before the intervention | 161.93 (40.2) | 163.24 (31.6) | 0.412 | |
| After the intervention | 147.54 (39.18) | 161.93 (32.02) | 0.072 | |
| EDWT, cm | 0.828 | |||
| Before the intervention | 1.17 (0.37) | 1.24 (0.39) | 0.869 | |
| After the intervention | 1.16 (0.37) | 1.23 (0.37) | 0.418 | |
| Vitamin D, ng/mL | < 0.001 | |||
| Before the intervention | 18.05 (7.48) | 16.85 (8.08) | 0.489 | |
| After the intervention | 49.05 (9.94) | 19.27 (8.59) | < 0.001 | |
| Albumin, mg/dL | 0.036 | |||
| Before the intervention | 3.99 (0.52) | 4.09 (0.43) | 0.350 | |
| After the intervention | 4.11 (0.48) | 4.11 (048) | 0.982 | |
| Creatinine, mg/dL | 0.442 | |||
| Before the intervention | 1.20 (0.3) | 1.15 (0.27) | 0.442 | |
| After the intervention | 1.22 (0.31) | 1.16 (0.27) | 0.532 | |
| Calcium, mg/dL | 0.827 | |||
| Before the intervention | 9.73 (0.44) | 9.71 (0.45) | 0.827 | |
| After the intervention | 9.77 (0.47) | 9.70 (0.47) | 0.821 | |
| Phosphor, mg/dL | 0.486 | |||
| Before the intervention | 3.50 (0.74) | 4.01 (0.64) | 0.486 | |
| After the intervention | 3.61 (0.67) | 4.11 (0.71) | 0.629 |
Abbreviations: EDV, end-diastolic volume; EDWT, End-diastolic wall thickness; EF, ejection fraction.
| Variable | Intervention Group | Placebo Group | P Value |
|---|---|---|---|
| Improvement of NYHA class | < 0.001 | ||
| Class ΙΙΙ to ΙΙ | 4 (9.7) | 1 (2.4) | |
| Class ΙΙ to Ι | 17 (41.5) | 7 (17.1) | |
| Without change | 20 (48.8) | 33 (80.5) |
Abbreviation: NYHA class, New York Heart Association Functional Classification.
aValues are expressed as No. (%)
Since none of the baseline variables were significantly different between the two groups and the levels of significance for all variables were above 0.2, multivariate analyzes were not performed.
5. Discussion
The results of this study showed that the ejection fraction of the patients was significantly improved after taking vitamin D compared to the group that did not take it. The results also showed a significant improvement in the left ventricular diastolic volume in the vitamin D-receiving group.
In a study that was consistent with the results of our study, Witte and colleagues reported a significant improvement in the ejection fraction of patients following vitamin D complementation. However, they failed to show a significant difference in the 6-minute walk distance test. The results of our study showed a significant difference in NYHA class between the two groups, which is contrary to the results of the Witte et al. (8) study. Part of the difference may be because of how the outcome is measured in the two studies. Although both the NYHA class and the 6-minute walk test are suggested to assess the functional status of patients with heart failure, the type of assessment in these two tests is different. Although patients in the NYHA class are evaluated based on symptoms and non-objective evaluations, evaluations on a 6-minute walk are more objective and based on patients’ ability to perform physical activity. However, this test can be affected by some confounders such as comorbidities that affect physical activity. On the other hand, it may not be unexpected that following the improvement in patients’ ejection fraction, patients’ functional and clinical status will also improve (18).
The effect of vitamin D on cardiac function improvement in patients with vitamin D deficiency can be expected. Vitamin D deficiency can be effective in cardiac remodeling in two main ways, so its deficiency leads to the disruption of calcium ion transportation in myocytes (19). On the other hand, heart failure causes conditions where the cell becomes overloaded with calcium, which disrupts both the contraction and the relaxation of myocytes (20). Vitamin D deficiency can also lead to cardiomyocyte hypertrophy and inflammation of the interstitial tissue, and fibrosis. Therefore, vitamin D deficiency can lead to the rapid progression of heart failure through adverse cellular damage and remodeling (21).
Undesirable remodeling, however, is mostly caused by increased activity of the renin-angiotensin-aldosterone system (RAAS) (which causes myocyte dysfunction and loss of myocyte and interstitial tissue fibrosis) (22). Inhibition of this process can cause reverse remodeling. As vitamin D deficiency can intensify the activity of this system, supplementation of vitamin D may reduce renin production and decrease its plasma activity (23).
According to a study by Boxer et al. (24), vitamin D has no effect on the outcome of patients with heart failure. These results are not in line with the results of the present study. One of the reasons for this difference may be differences in the study population by age, NYHA class, vitamin D prescription dose, and how the outcomes were assessed in the two groups. Although the present study included over 40 years old patients as the study population, the Boxer et al. (24) study population was over 50 years old. This can affect the response rate to the treatment so that older people may respond inappropriately to the interventions. On the other hand, in our study, individuals with NYHA class 1 to 3 were evaluated, and subjects with class 4 disease were excluded. In the Boxer et al. (24) study, subjects from grades 2 to 4 were evaluated. It may be predictable that individuals with a lower level of performance will respond less favorably to the interventions. The present study was able to partially control the impact of confounding factors in this area by limiting patient selection among functional classes of 1 to 3. Another difference can be attributed to the amount of vitamin D intake in the two studies, so we considered higher doses for patients in this study that may enforce the role of dose-dependent factor associated with vitamin D treatment. However, confirmation of this issue would require further dose-dependent studies. On the other hand, Boxer et al. (24) evaluated patients’ primary outcome based on VO2 peak through cardiopulmonary testing, whereas changes in heart rate and diastolic end volume were considered the major outcomes of the present study.
Our study revealed a statistically significant difference between the two groups in terms of end-diastolic volume changes, which seems to be important clinically. In our research, the amount of this volume was 147.54 (39/18) in the intervention group and 161.93 (32/02) in the placebo group (with a significance level of 0.072). The relatively greater effect of the intervention on the ejection fraction than the end-diastolic volume may be attributed to the effects of vitamin D on the improvement of ventricular contractions in addition to reversed ventricular remodeling. This would cause the ejection fraction, which depended on both end-diastolic volume and end-systolic volume, to have better effects than just the end-diastolic volume (21). Study duration can also be effective, as the remodeling phenomenon may require more time. Finally, our study showed that vitamin D had no effect on the thickness of the ventricular wall, which was not unexpected. Because, as mentioned above, vitamin D can exert its positive effects through its effects on ventricular contractions and remodeling phenomena (processes that will not affect ventricular wall thickness), and not through the impact on myocyte size.
In the present study, the effect of vitamin D on serum albumin was also investigated. The results of this study showed that the vitamin D-receiving group had a higher increment in serum albumin level than the control group. This can be a consequence of the effect of vitamin D on heart failure of the patients, thereby improving renal function and gastrointestinal status (by lowering central venous pressure), which results in an increase in serum albumin (25).
In this study, the effect of vitamin D on the outcomes of patients with heart failure was also investigated. One of the benefits of this study is to evaluate the impact of this intervention on both objective outcomes, such as patients’ ejection fraction, and non-objective outcomes, such as the NYAH class. The results of this study showed that taking vitamin D could have a positive effect on both types of outcomes. Nevertheless, our study had some limitations. One of the limitations of this study is the time of the patient’s follow-up. Longer follow-up of patients could yield more definite results. However, longer time that can increase confounding factors such as the need for revascularization, hospitalization, and changes in medications of patients, as well as overtime increasing of creatinine, were excluded from this study. The incidence of some comorbidities can also affect the final outcome of patients. Another limitation of the current study is the small sample size. However, the sample size was calculated based on a second type error of 20% and a first type error of 5%. On the other hand, the power analysis of the post-study showed a power of 0.7912, which was approximately 80% and could be considered appropriate. We tried to control vitamin D sources, including refraining from sunbathing, offering the same diet, especially for dairy and legumes, not taking vitamin D outside the proposed program. Although we tried to control vitamin D sources, one of the limitations of the study during the follow-up was the inability to complete the control of vitamin D sources for patients such as sunlight exposure (through which is produced 90% of the body’s vitamin D), physical activity, food intake, and the other source of vitamin D.
Although objective and non-objective outcomes, including ejection fraction, end-diastolic volume, serum level of albumin, and NYHA grade were evaluated in this study, outcomes such as annual mortality and annual rehospitalization rates were not evaluated. Investigation of these consequences will require a longer time, which is not the primary purpose of this study.
5.1. Conclusions
The results of the current study indicate that supplementation of vitamin D can be effective in the improvement of left ventricular ejection fraction and functional ability of vitamin D-deficient patients. If more comprehensive and generalizable studies conducted on the normal population support this hypothesis, vitamin D deficiency assessment and correction in patients with chronic heart failure may be recommended.