1. Background
Diabetes mellitus has emerged as a major epidemic today (1). There is a need for a rapid, easily available, non-cumbersome, reproducible, reliable, non-fasting test for diagnosing diabetes as well as for screening. Considering these objectives, the American Diabetes Association recommended glycated hemoglobin (HbA1c) as a diagnostic test for diabetes mellitus. The diagnosis threshold of HbA1c for pre-diabetes is ≥ 5.7% but < 6.5%, whereas a value ≥ 6.5% was recommended for diabetes mellitus (2, 3).
Glycated hemoglobin is the percentage of hemoglobin that has undergone non-enzymatic glycation while the red blood cells (RBCs) are in circulation (4, 5). Although it reflects the mean glucose levels over the preceding 2 - 3 months, it also depends on the average duration of exposure of the circulating erythrocytes to this glucose (6). Diseases associated with a low erythrocyte turnover have a preponderance of older RBCs, which are associated with a falsely high HbA1c. Iron and vitamin B12 deficiency (6) and renal failure (7) are examples. Conversely, treatment of iron and vitamin B12 deficiency (6) and administration of erythropoiesis-stimulating agents (8) in renal failure lowers the previously elevated HbA1c, without any impact on glucose.
Falsely elevated HbA1c is observed in hypothyroidism where erythropoiesis is usually sluggish (9). Hyperthyroidism has the opposite effect of increasing RBC turnover (increase in erythropoiesis accompanied by a shortened RBC survival time) (10), thereby causing a potential reduction in HbA1c relative to the level of glycemia. There are, however, conflicting reports of high (11, 12) and low HbA1c (13) in hyperthyroidism compared to matched controls. But these studies were cross-sectional in nature. One (possibly underpowered) study (14) has found no changes in HbA1c after treatment of hyperthyroidism. This needs corroboration in a larger study. We hypothesize that HbA1c levels may not truly reflect the level of ambient glycemia in hyperthyroidism due to changes in RBC turnover.
2. Objectives
To assess longitudinally, the impact of medical correction of hyperthyroidism on glycated hemoglobin, independent of changes in plasma glucose.
3. Methods
The study was a prospective longitudinal before-after intervention study conducted in the Department of Endocrinology at the Sri Venkateswara Institute of Medical Sciences, Tirupati. The study period was from April 2017 to September 2018. The protocol was approved by the Institutional Ethics Committee. Adults ≥ 18 years with overt hyperthyroidism i.e., thyroid-stimulating hormone (TSH) < 0.1 µIU/mL and thyroxine (T4) > 12.23 µg/dL were invited to take part in the study after obtaining written informed consent. Patients with overt diabetes mellitus [based on their fasting plasma glucose (FPG) being ≥ 126 mg/dL or a post 75 gm oral glucose tolerance test (OGTT) two-hour ≥ 200 mg/dL (7) or being on pharmacological treatment for diabetes mellitus] were excluded from the study. HbA1c was not used to define diabetes.
Also, pregnant women, patients with chronic kidney disease (CKD) or those known to have abnormal hemoglobinopathy, hemolytic disorder, reticulocytosis (> 2.5%) attributable to a co-existing disorder, or bone marrow disorders such as aplastic anemia or myelodysplastic syndrome or a recent (< 3 months) blood transfusion were excluded.
The weight and height were measured. Patients were phlebotomized between 0800 - 0900 am in fasting state for plasma glucose, hemoglobin (Hb), reticulocyte percentage, creatinine, tri-iodothyronine (T3), thyroxin (T4), and thyrotropin (TSH). A standard oral glucose tolerance test with glucose (equivalent to 75 gm anhydrous glucose), was then performed and samples for plasma glucose were collected after two hours.
T3, T4, and TSH were measured by automated chemiluminescence immunoassay (Access II Beckman Coulter Inc. CA, USA). Details of the immunoassay kits are given in Table 1.
| Analyte | Assay Sensitivity | Coefficient of Variation %, (High Level Control - Low Level Control) | Normal Range |
|---|---|---|---|
| T3 (ng/dL) | 10 | 4.74 - 9.12 | 87 - 178 |
| T4 (µg/dL) | 0.50 | 4.01 - 6.63 | 6.09 - 12.23 |
| TSH (mIU/L) | 0.003 | 3.72 - 4.96 | 0.34 - 5.6 |
HbA1c was estimated by ion-exchange high-pressure liquid chromatography on D-10 Hemoglobin testing system, Bio-Rad Laboratories, Hercules, California, United States). This technique has a within-run coefficient of variation (CV) of 0.78% and a between-run CV% of 0.52% at a mean HbA1c of 5.7% in non-diabetic subjects. Glucose and creatinine were measured on an autoanalyzer: Unicel DXC 600 Synchron Clinical Systems (Beckman Coulter, Galway, Ireland). Glomerular filtration rate was estimated by using the MDRD formula (15). Hemoglobin was estimated on an automated hematology analyzer by colorimetric method (Shenzhen Mindray Biomedical Electronics Co Ltd, Hamburg 20537, Germany). A peripheral blood smear was stained with methylene blue and the reticulocytes were identified and reported as a percentage of the total RBC count.
Patients were then started on an appropriate dose of Carbimazole and periodically followed up with a regular estimation of total T4 till it was nearly in the normal range. The dose of Carbimazole was then reduced as required (based on frequent monitoring of T4) to maintain a near euthyroid state for a further three months. Thereafter the following investigations were repeated as before: serum T3, T4 and TSH, FPG and post OGTT two-hour plasma glucose, HbA1c, Hb, and reticulocyte percentage.
3.1. Data Analysis
Continuous variables were expressed as mean ± SD if they were normally distributed and as median (25th percentile – 75th percentile) if not normally distributed. Comparison of means between baseline and the post-treatment values were performed by paired t-test for normally distributed data and by Wilcoxon Ranked Sum Test for others. Categorical variables were compared between baseline and post-treatment points of time by McNemar Chi-square test for paired data. A P-value ≤ 0.05 was taken as significant.
The sensitivity and specificity of HbA1c at the cut-off ≥ 5.7% to diagnose prediabetes was determined using a combination of fasting and two hours post OGTT plasma glucose as the gold standard (100 ≤ FPG < 126 mg/dL OR 140 ≤ two hours post OGTT plasma glucose < 200 mg/dL OR both). Sensitivity was the percentage of patients with prediabetes on glucose criteria who had HbA1c ≥ 5.7 %, while specificity was the percentage of patients with normal glucose who had HbA1c < 5.7%.
Sensitivity = (Number of patients with prediabetes on glucose criteria having HbA1c ≥ 5.7)/(Total number of patients with prediabetes on glucose criteria )×100
Specificity = (Number of patients with normal glucose in both fasting and post OGTT having HbA1c< 5.7%)/(Total number of patients with normal glucose in both fasting and post OGTT)×100
4. Results
Forty-six consecutive patients with hyperthyroidism were initially invited to take part. Of these, one patient was diagnosed to be having diabetes mellitus (based on OGTT) and therefore was excluded, while eight patients were lost to follow-up. One patient became pregnant and was hence excluded. Thirty-six patients completed three months of follow up after achieving the euthyroid state and were available for repeating the evaluation.
Mean age of these 36 patients was 43.0 ± 12.2 years. Twenty-seven (75%) were female and 9 (25%) males with a female to male ratio of 3:1. Mean weight and body mass index (BMI) was 50.9 ± 9.2 kg and 20.1 ± 3.1kg/m2 at baseline. Mean T3 and T4 at the time of recruitment were 320 ± 160 ng/dL and 21.82 ± 5 ng/mL respectively. TSH [median (1st to 3rd quartile)] was 0.01(0.01 - 0.03) µIU/mL. Hemoglobin was 12.2 ± 1.3gm/dL and reticulocyte percentage [median (1st to 3rd quartile)] at baseline was 1 (0.5 - 1.8) %.
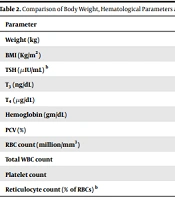
Patients were documented to become nearly euthyroid at a median of 96 days (8 days interquartile range) from recruitment, initial baseline sampling and the commencement of Carbimazole treatment. Patients were then followed up and re-assessed a further median 129 days (interquartile range of 46 days) after they initially became euthyroid. T3, T4, at this time was 107 ± 33 ng/dL and 8.08 ± 2.09 µg/dL, respectively while the TSH [median (1st to 3rd quartile)] was 0.45 (0.03 - 2.8) µIU/mL. There was an increase in the mean body weight by 6.3kg (P < 0.001) (Table 2). Following treatment of hyperthyroidism, there was a rise in the hemoglobin from 12.2 ± 1.2gm/dL to 12.8 ± 1.4 gm/dL (P = 0.004) along with an improvement in the packed cell volume (P = 0.012). There was no change in the reticulocyte percentage (P = 0.66) (Table 2).
| Parameter | Pre-Therapy | Post-Therapy | P-Value |
|---|---|---|---|
| Weight (kg) | 50.9 ± 9.2 | 57.3 ± 9.3 | < 0.001 |
| BMI (Kg/m2) | 20.1 ± 3.1 | 22.61 ± 3.02 | < 0.001 |
| TSH (µIU/mL) b | 0.01 (0.01 - 0.03) | 0.45 (0.03 - 2.8) | < 0.001 |
| T3 (ng/dL) | 320 ± 160 | 107 ± 33 | < 0.001 |
| T4 (µg/dL) | 21.82 ± 5 | 8.08 ± 2.09 | < 0.001 |
| Hemoglobin (gm/dL) | 12.2 ± 1.2 | 12.8 ± 1.4 | 0.004 |
| PCV (%) | 38.1 ± 4.4 | 39.6 ± 4.3 | 0.012 |
| RBC count (million/mm3) | 4.45 ± 0.36 | 4.57 ± 0.64 | 0.148 |
| Total WBC count | 6816 ± 1664 | 7158 ± 1536 | 0.215 |
| Platelet count | 2.6 ± 0.6 | 2.7 ± 0.7 | 0.099 |
| Reticulocyte count (% of RBCs) b | 1 (0.5 - 1.8) | 1 (0.5 - 2) | 0.66 |
Abbreviation: PCV, packed cell volume.
aNormal value: T3, 87 - 178 ng/dL; T4, 6.09 - 12.23 µg/dL; TSH, 0.34 - 5.6 µIU/mL; Reticulocyte, 0.5 - 1.5%.
bThese parameters were expressed as median (1st - 3rd quartile) and the comparison of these parameters between pre- and post-therapy time periods were done by Wilcoxon ranked sum test as they were not normally distributed.
4.1. Changes in Glycemic Indices
After achieving a near euthyroid status, the mean fasting plasma glucose (FPG) and post 75gm OGTT two-hour plasma glucose was 87.7 ± 15.4 mg/dL and 116.6 ± 22.6 mg/dL, respectively. These were not significantly different from baseline values (Table 3).
| Parameter | Pre-Therapy | Post-Therapy | P-Value |
|---|---|---|---|
| FPG (mg/dL) | 84.9 ± 10.3 | 87.7 ± 15.4 | 0.28 |
| Post OGTT 2-hour plasma glucose (mg/dL) | 120 ± 26.7 | 116.7 ± 22.6 | 0.54 |
| HbA1c (%) | 5.20 ± 0.37 | 5.35 ± 0.45 | 0.025 |
Abbreviation: FPG, fasting plasma glucose; HbA1c, Glycated hemoglobin; OGTT, oral glucose tolerance test.
Mean HbA1c at baseline was 5.20 ± 0.37%. After achievement of euthyroid status the HbA1c rose to 5.35 ± 0.45% (P = 0.025, Table 3). Given the age of the patients or changes in hemoglobin levels, T4 or body weight were not independent indicators of change in HbA1c using multiple linear regression analysis.
There was a significant increase in the proportion of patients with HbA1c ≥ 5.7 % from 3/36 (8.3%) in hypothyroid phase to 10/36 (27.7%) in euthyroid phase (P = 0.016). However, the proportion of patients with prediabetes based on glucose measurements (not on HbA1c) i.e., 100 ≤ FPG < 126 mg/dL OR 140 ≤ 2-hour post OGTT plasma glucose < 200 mg/dL OR both, did not change after treatment (10/36 (27.7%) before treatment vs. 8/36 (22.2%) after treatment, P = 0.50).
The sensitivity and specificity of HbA1c at the cut-off ≥ 5.7% to diagnose prediabetes was determined using a combination of fasting and 2-hour post OGTT plasma glucose as the gold standard (100 ≤ FPG < 126 mg/dL OR 140 ≤ 2-hour post OGTT plasma glucose < 200 mg/dL OR both). Sensitivity was low (20%) and specificity high (96%) during the hyperthyroid phase. After correction of hyperthyroidism, the sensitivity rose to 50%, while specificity fell to 78%.
5. Discussion
The American Diabetes Association has approved the use of HbA1c for the diagnosis of diabetes mellitus, subject to the requirement that the HbA1c testing must be standardized as per the National Glycohemoglobin Standardization Program (NGSP) (16) .
The diagnostic threshold of HbA1c for diabetes was set at 6.5%, while normal HbA1c was defined as values below 5.7% (7). Values greater than or equal to 5.7% but < 6.5% were considered to identify a group with an intermediate level of dysglycemia namely the prediabetic state (7).
Disorders characterized by altered erythrocyte turnover can cause HbA1c perturbations independent of the glycemic status by changing the relative percentages of young and old erythrocytes in peripheral blood, thereby affecting the mean age of the RBCs in circulation (17). Thus a rapid turnover of RBCs as seen in hemolytic anemia (18) or acute blood loss can falsely lower the HbA1c; however, disorders associated with reduced RBC turnover such as iron or cobalamin deficiency,6 or chronic kidney disease (7) with low glomerular filtration rate can have a falsely elevated HbA1c.
One of the conditions associated with altered RBC turnover is an abnormal thyroid status. regarding hypothyroidism, previous studies have shown reduced turnover. The erythrocyte lifespan remains normal but there is hypoproliferative erythropoiesis (19, 20), which is mainly attributed to the relative deficiency of erythropoietin (19). Consequently, younger RBCs are reduced in their proportion in the peripheral blood, leading to a preponderance of older RBCs. This leads to an elevated HbA1c relative to the level of glycemia (19, 21).
A condition opposite to this is hyperthyroidism. In this condition, there is enhanced erythropoiesis (possibly related to increased erythropoietin) accompanied by a shortened RBC survival time (22). A faster turnover would be expected to reduce the average age of RBCs in circulation, thereby causing a false lowering of HbA1c in relation to the level of glycemia. As studies on the effect of hyperthyroidism and its treatment on HbA1c are sparse, we planned to undertake this study in non-diabetic hyperthyroid patients.
Newly diagnosed patients with overt primary hyperthyroidism (T4 > 12.23 µg/dL and TSH < 0.1 µIU/mL)
were enrolled. Patients with plasma glucose in the diabetic range (i.e., FPG ≥ 126 mg/dL and 2-hour post OGTT plasma glucose ≥ 200 mg/dL) were excluded, because any anti-hyperglycemic treatment required to be given to them for glycemic control would have changed the glucose values independent of hyperthyroidism control alone. Whereas our aim was to document changes in plasma glucose and HbA1c resulting from the treatment of hyperthyroidism alone.
On restoration of euthyroid status with Carbimazole, the dose was reduced as required to maintain a near euthyroid state for a further three months. We chose three months for the reassessment of the patients due to the approximately 120-day lifespan of the RBCs in the circulation.
5.1. Changes in Hematological Indices After the Treatment of Hyperthyroidism
In the present study, after restoration of euthyroidism, there was a rise in the hemoglobin (P = 0.004). An increase in hemoglobin, on treatment of hyperthyroidism was also reported previously (22, 23). Bhattacharjee et al.(14) showed a significant decrease in reticulocyte count after treatment, indicating a lower turnover after treatment. The high reticulocyte count in hyperthyroidism could be the effect of elevated erythropoietin- a reflection of the increasing demand of the hyper metabolizing tissues for oxygen (10). However, there was no change in reticulocyte percentage in our study following the treatment of hyperthyroidism.
5.2. HbA1c in Hyperthyroid Patients at Baseline and After Restoration to a Near Euthyroid State
The HbA1c in our patients with non-diabetes range glucose values during the phase of hyperthyroidism was 5.2 ± 0.37 %. In a comparable study from general Indian population, the HbA1c in healthy patients with normal fasting glucose was slightly higher at 5.4 ± 0.04% (mean ± SD) (24).
In the present study, although post-treatment there was an increase in the HbA1c to 5.35 ± 0.45% (a closer approximation to the values found in euglycemic general Indian population noted above), there were no corresponding changes in the mean fasting and the 2- hour post OGTT plasma glucose. Also, the proportion of patients with HbA1c ≥ 5.7% (prediabetes by HbA1c criteria) rose following treatment. This was not accompanied by any significant increase in the proportion of prediabetes patients based on plasma fasting glucose values and 2-hour post OGTT plasma glucose values.
This implies that during the phase of thyrotoxicosis, the HbA1c must have been falsely low in relation to the level of glycemia and its rise (despite no change in plasma glucose) following treatment probably represents a restoration to normal. This is indeed expected in conditions associated with a high RBC turnover as seen in thyrotoxicosis (10).
However, studies on HbA1c in thyrotoxicosis are not consistent. Two studies reported that HbA1c was higher in hyperthyroid patients compared to euthyroid controls; however, one study had a higher fasting plasma glucose (12) in hyperthyroid patients, the other showed a trend toward higher mean plasma glucose (11) in hyperthyroid group. Thus, the higher HbA1c in hyperthyroid patients could be easily attributed to the higher glycemia in the hyperthyroid group of patients. These were cross-sectional studies.
The present study is a longitudinal study, wherein, there were no changes in plasma glucose after treatment compared to the baseline. Despite that the HbA1c increased, which is a unique observation.
Bhattacharjee et al.(14) studied the effect of treatment with anti-thyroid drugs on HbA1c in patients with Graves’ disease. Treatment did not significantly change the median HbA1c value, despite a significant reduction in reticulocyte count. But their study included only 20 patients and might have been underpowered.
5.3. Diagnostic Performance of HbA1c to Diagnose Prediabetes in Hyperthyroidism
A study from Fuzhou (13) in China showed that the percentage of Graves’ disease patients with abnormal glucose metabolism (prediabetes and diabetes mellitus) as defined by HbA1c levels was lower than that by OGTT criteria. The HbA1c was less sensitive for diagnosing diabetes in Graves' disease (34.9%) than in patients with euthyroid goiter (62.3%) and healthy controls (60.6%); however, it was shown to be equally specific (99.3%, 98.6%, 97.4% in patients with Graves’, euthyroid goiter and healthy controls respectively). As a cross-sectional study, their results are further borne out by the present longitudinal study as the sensitivity of HbA1c to diagnose pre-diabetes was lower (20%) when the patients were hyperthyroid than when the euthyroid state was restored (50%). Specificity was conversely higher both during the hyperthyroid (96%) and in the euthyroid phases (78%).
This is consistent with a falsely low HbA1c in relation to the prevailing glycemia-due to which its sensitivity was lower, during the thyrotoxic phase. Thus, fasting plasma glucose and OGTT and not HbA1c are the only reliable tests for prediabetes and presumably diabetes in hyperthyroidism.
The somewhat disappointing sensitivity of 50% and specificity of 78% for detection of prediabetes by HbA1c reported even after restoration to the euthyroid state is comparable to that reported by Nair et al. (24) from the general Indian population for the detection of prediabetes (impaired fasting glucose) of only 59.7% and specificity of 59.9%. The present study was limited by a small number of patients (n = 36), limited post-treatment follow-up (three months after euthyroidism) and the lack of both healthy as well as untreated hyperthyroid patients as controls.
As there was an increase in levels of glycosylated hemoglobin after treatment of hyperthyroidism, with no changes in the mean fasting or 2-hour post-OGTT plasma glucose, it may be concluded that HbA1c is falsely low in relation to glycemia in patients with hyperthyroidism. In view of its low sensitivity, it should not be employed as a test to make a diagnosis of diabetes or prediabetes in patients with hyperthyroidism. Only fasting plasma glucose and an OGTT should be used for this purpose.