1. Background
Type 1 diabetes mellitus (T1DM) is a chronic metabolic disorder caused by a deficiency of insulin, which contributes to hyperglycemia. The most common acute complication in T1DM is diabetic ketoacidosis (DKA), and it usually manifests with fatigue, ketotic breath, nausea, tachypnea, and abdominal pain. DKA, at one point in time, was considered a hallmark of T1DM but is now being diagnosed in a vast majority of youth onset T2DM. Recently, an increased number of patients with T2DM were presented with new-onset T2DM with DKA at diagnosis (1). A relatively recent subtype of diabetes, ketosis-prone diabetes (KPD), and DKA is the first presentation in the treatment of individuals with naive T2DM.
Winter et al. (2) first reported atypical or ketosis-prone T2DM way back in 1987. It has its own set of clinical features that differentiate it from other types of diabetes. In 2002, Sobngwi et al. (3) introduced the term “ketosis-prone diabetes” in a review of diabetes in West Africans. Ketosis-prone diabetes, also known as “Flatbush Diabetes”, has pathophysiology close to that of T2DM but initially exhibits signs and symptoms of T1DM (4). Patients with KPD frequently present with elevated levels of glucose of 500 -700 mg/dL, high levels of ketone, and hemoglobin A1C ranging from 12% to 14%. However, in contrast to many other cases of DKA seen in T1DM, many patients do not exhibit autoantibodies to beta cells. The resemblance with T1DM was observed in people with impaired insulin secretion, which could also lead to ketoacidosis when combined with severe insulin resistance.
According to the AB classification scheme, the individuals with new-onset diabetes exhibiting ketoacidosis in the absence of IA2 and GAD65 autoantibodies (A-) and complete functional recovery of beta-cells (β+) were defined to suffer from A-β+ KPD (5, 6). In contrast, those with ketoacidosis in the presence of IA2 and GAD65 autoantibodies (A+) but failure in functional recovery of beta-cells (β-) were defined as A+β-KPD (7). Between 1995 and 2003, KPD case series and retrospective reviews revealed a large number of patients from a broad range of geographical areas and ethnicities, which consisted of reports from Japanese, Apache Indians, diverse ethnic groups of United States demographics such as Hispanics, Caucasians, and Native Americans, Europeans, Pakistanis and Chinese (4). However, data reported in India are very limited.
2. Objectives
In this study, we report six cases of KPD that followed a set pattern starting from DKA presentation, followed by high dose insulin requirement, and in due course of time, all the patients completely stopped insulin and exhibited ‘remission from insulin dependency’. Fasting plasma glucose < 124 mg/dL and HbA1c ≤ 6.3% at three-month post-insulin discontinuation defined remission (8).
3. Methods
This observational, descriptive, prospective, non-interventional study consisted of six DKA cases of either gender with age ranging from 14 - 65 years that were followed up for 24 months from the index episode of DKA. Ethics committee approval was obtained from the Institutional Ethics Committee.
All of the patients with DKA admitted from June 2016 to June 2019 were evaluated using a standard protocol by the same observer. Eligible cases had to fulfill all the prerequisites: new-onset diabetes with ketoacidosis, no precipitating factors (infections; acute cerebrovascular accident; acute myocardial infarction; pancreatitis; not on any known drugs that may precipitate DKA, like thiazides and steroids). After sorting out 96 cases of DKA admitted during the study period, we were able to clinically recognize six cases (6.2%) as KPD and followed them for 24 months. We had used “AB” classification for the diagnosis of patients with KPD.
The demographic characteristics for this study consisted of patients’ age, gender, family history of diabetes, history of co-morbid illness, symptoms at the time of presentation along with the duration of symptoms, prior treatment, consumption of drugs, and the presence of precipitating factors of DKA (stress, infections, etc.). All patients were examined, with special attention to the presence or absence of markers of insulin resistance-skin tags and acanthosis nigricans, along with the examination of peripheral pulses. Laboratory tests included Glycated hemoglobin (HbA1c), Serum C-peptide, Blood Sugar, and other routine investigations. IA2 and GAD65 autoantibodies were quantified using the Quantitative Sandwich Immunoassay method (RSR Limited, Cardiff, UK) with a detection range from 5 -2000 U/mL.
4. Results
The mean age of the patients was 30.83 ± 16.72 years. The male:female ratio was 5:1. Four of the six patients presented with symptoms of polyuria and polyphagia over a period of 2 to 4 weeks with weekly weight loss of 1.4 ± 1.0 kg. Five patients had a family history of diabetes. Clinical characteristics of the presentation have been mentioned in Table 1. The mean BMI was found to be 28.65 ± 2.94 kg/m2. All patients presented with grade II-III acanthosis nigricans. Skin tags were seen in three patients. No target organ damage was diagnosed in any of the patients at presentation.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | |
|---|---|---|---|---|---|---|
| Age, y | 32 | 14 | 29 | 16 | 65 | 29 |
| Gender | Male | Male | Male | Female | Male | Male |
| Height, cm | 162 | 173 | 168 | 160 | 170 | 176 |
| Weight, kg | 92 | 82 | 74 | 72 | 80 | 85 |
| BMI, kg/m2 | 35.1 | 27.4 | 26.2 | 28.1 | 27.7 | 27.4 |
| Acanthosis nigricans (8) | Grade 3 | Grade 3 | Grade 3 | Grade 3 | Grade 2 | Grade 3 |
| Skin tags | Yes | No | Yes | No | No | Yes |
| Duration of symptoms, wk | 2 | 4 | 3 | 4 | 3 | 3 |
| Polyuria | Yes | Yes | Yes | Yes | No | No |
| Polyphagia | Yes | Yes | Yes | Yes | No | No |
| Weight loss | 5 kg in 2 weeks | 6 kg in 4 weeks | 4 kg in 4 weeks | 4 kg in 4 weeks | 4 kg in 4 weeks | 5 kg in 4 weeks |
| Other medical history | Graves’ disease | None | None | Hypothyroidism (new diagnosis) | Hypertension | Sore throat 2 weeks ago |
| Precipitating factor for DKA | None | None | None | None | None | None |
| Family history of diabetes | Father at 54 years; mother at 45 years; one elder sibling at 40 years | Mother at 35 years | Nil | Father at 52 years | Brother at 62 years; SCD at 65 | Mother at 39 years; father at 44 years |
Clinical Characteristics at Presentation
Renal and hepatic functions were normal at the presentation in all the cases. All patients exhibited severe ketoacidosis at presentation. The C-peptide assay was done six weeks after the index DKA episode. The mean C-peptide level was 2.35 ng/dL (done 4 - 6 weeks after the index episode of DKA with FBS < 130 mg/dL), which indicated good beta-cell reserve. All cases were negative for IA2, and GAD65 autoantibodies hence belonged to the A-β+ category. Detail biochemical presentation has been described in Table 2.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | |
|---|---|---|---|---|---|---|
| GRBS, mg/dL | HI | 434 | 553 | 459 | 559 | HI |
| HbA1C | 14.3 | 11.2 | 10.8 | 14.1 | 14.1 | 16.2 |
| pH | 6.9 | 7.1 | 7.1 | 7.05 | 6.9 | 6.9 |
| Bicarbonate | 3.0 | 10 | 8.03 | 10.4 | 5.06 | 9.08 |
| UKB | + | + | + | + | + | + |
| GAD antibodies | - | - | - | - | - | - |
| IA2 Antibodies | - | - | - | - | - | - |
| Fasting C-peptide (AFTER 6 weeks of presentation), ng/mL | 2.4 | 3.2 | 2.1 | 2.4 | 2.15 | 1.9 |
Biochemical Characteristics at Presentationa
As shown in Table 3, the patients presenting with DKA were initially treated with high doses of insulin, ranging from 90 - 120 units/day, mean insulin requirement: 100.3 units/day. All patients were discharged on a basal-bolus or a modified basal-bolus regimen (three pre-meal premix insulin or two short-acting and one premix dose before dinner).
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | |
|---|---|---|---|---|---|---|
| IV dose of insulin, units/day | 108 | 84 | 120 | 90 | 104 | 96 |
| S/C inulin dose at discharge | A-32-26-26; L-36 | A-24-24; HM-30 | A-22-22-0; M-0-0-28 | NM; 26-24-26 | A-24-24-20; L-0-0-0-24 | HM; 30-16-30 |
| The period required to stop insulin, wk | 6 | 3 | 4 | 3 | 4 | 3 |
| Current medicines (Two years since diagnosis) | Metformin (500 BD); sitagliptin (50 BD) | No OHA | Lost to follow-up | Metformin (500 OD) | Glimeperide (1 mg OD); metformin (500 BD) | Glimeperide (1 mg OD); metformin (500 BD) |
Insulin Requirements and Follow-up
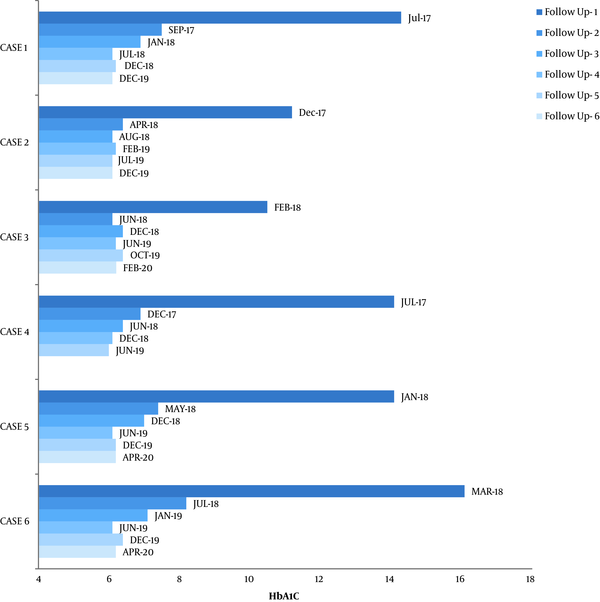
By the end of three months from the index episode, insulin administration was stopped for all six cases, and they were able to maintain normoglycemia with the administration of metformin at 1,000 mg/day in two divided doses in most cases. One case required Sitagliptin 100 mg, and two others required glimepiride 1 mg to maintain normoglycemia. None of the patients had DKA recurrence during the two-year follow-up. During this follow-up, none of the cases required insulin again to maintain euglycemia. Insulin was not required again to maintain glycemia. HbA1C was monitored at regular intervals, as shown in Figure 1. Throughout the follow-up, we observed an HbA1C decrease in all six cases.
5. Discussion
The recruited cases followed a set pattern of very high insulin requirement at diagnosis. On follow-up, the insulin requirement progressively declined, and all of the cases were able to stop insulin therapy after a mean period of four weeks. Initially, KPD was believed to be restricted to the African-Americans only; however, eventually, there were reports on KPD occurrence in other ethnic groups such as Chinese, Hispanic, South Asians, sub-Saharan Africans, and Caucasians (7, 9). There have been several studies that demonstrated, based on their ethnicity, individuals might be genetically predisposed to KPD. For instance, the HNF-1 gene has been considered to be a genetic marker of KPD in African American individuals; however, sub-Saharan and Afro-Caribbean ethnic groups do not exhibit any such association (10). To the best of our knowledge, our case series is the second reported case series on Indian patients with KPD, after the study of Gupta et al. (5).
We analyzed the clinical presentation and tried to determine a set of clinical and biochemical features that may differentiate KPD from other forms of DKA. Our cohort of patients showed a male predominance, severe DKA at presentation. The whole cohort followed a set pattern. The patients required insulin > 1.5 units/kg at the initial presentation. Over a period of four weeks, we observed a gradual fall in the insulin requirement. Eventually, the patients were able to get off insulin and maintain normoglycemia. This clinical representation was in agreement with the AB classification.
According to previous studies (2, 11), there is pediatric preponderance in age. However, in this study, the mean age of the patients was 30.83 ± 16.72 years (ranging between 14 - 65 years). Previous studies have shown that A-β+ KPD predominantly occurs in males (10). The same is reflected in our findings with a male:female ratio of 5:1. In our cohort, the mean BMI was 28.65 ± 2.94 kg/m2. In the Chinese cohort (9), the mean BMI of atypical diabetes mellitus patients was comparable to that observed for the patients in our study. Other studies, by Umipierrez et al. (12) and Smiley et al. (8), showed similar results. Five out of six patients (83%) exhibited a family history of diabetes, which was similar to the percentages reported in previous studies (10, 13). Previous studies have also reported that KPD is more strongly associated with a family history of diabetes than in cases of T1DM (10).
In this study, we observed that most of the cases (67%) exhibited symptoms for an average of 3 weeks. Our findings were similar to those reported by Mauvais-Jarvis et al. (10), who reported that the patients remained symptomatic for < 4 weeks. The clinical findings at presentation used to assess the markers of insulin resistance included acanthosis nigricans and skin tags. We observed acanthosis nigricans in all the cases and skin tags in 50% of the cases. Previously, Balasubramanyam et al. (4) also reported similar findings.
The biochemical profile and acid-base parameters of our patients were similar to those reported by Smiley et al. (8). The mean glucose and Hb1Ac levels were 501 mg/dL and 13.4%, with an average pH of 6.9. The high value of HbA1C indicates hyperglycemia that remained undiagnosed for a long time either due to weak symptoms or due to delay in the treatment owing to fewer resources or patients seeking care for their symptoms (5). The beta-cell assessment was done by evaluating the C-peptide levels at six weeks post index DKA episode. The mean C-peptide level was 2.35 ng/mL (1.9 - 3.2 ng/mL). In the present study, the recruited cases were managed by standard treatment protocols and discharged while being subjected to varying insulin regimen and doses. Previous studies have shown that insulin administration could markedly contribute to the functional recovery of beta-cells (5).
Previous studies have shown that, soon after discharge from the hospital, patients with KPD can successfully withdraw from insulin therapy after a few weeks, which is attributed to the correction of the acute glucotoxicity and subsequent functional recovery of beta-cells (13-18). All of our patients were initially managed with high intravenous insulin doses (1.8 - 2.4 units/kg). The time required for achieving remission varied widely. The least remission period was 10 weeks, and the longest time till remission was 16 weeks. A similar study conducted by McFarlane et al. (15) reported a mean remission time of 83 days for 42.3% of the patients in their cohort. None of our patients exhibited DKA recurrence during the follow-up for two years. We selected the period of two years because previous studies have shown recurrence of A-β+ KPD within 2 years of diagnosis (10).
The major strengths of this study included its prospective nature and long-term and real-time analysis , long-term follow-up of the patients and real-time analysis. The major limitation of our study included lack of mixed meal test (MMT) that is generally employed for an appropriate assessment of the function of beta cells. Another limitation of this study was that we did not analyze the ZnT8 antibodies, which at times, can be the only hallmark of T1DM. The results of the current study can be beneficial to confirm the presence of KPD in general and A-β+ type in particular. Given the immense rise in the incidence of diabetes, our findings could also be helpful in determining the assessment approach and drawing appropriate inferences from observations for future studies and may help in elucidating the pathology of this rare condition.
In resource-poor settings with the lack of easy availability of assays to perform quality antibody tests, coupled with the exuberant costs of the assays, in developing countries like India, the above clinical picture should arise a clinical suspicion of A-β+ type of KPD, wherein proper and regular follow-up can be done with patient on insulin, antibody assays can be analyzed at a later point in time. Insulin needs to be continued for a period of two to four weeks with regular self-monitoring of blood glucose at home, thereafter insulin can be tapered and stopped.
5.1. Conclusions
Ketosis-prone diabetes is often under-recognized and under-reported among all types of diabetes. The recognition is of utmost importance as the approach and treatment vary widely from the conventional type of diabetes. Proper follow-up, especially in unprovoked cases of DKA with obese phenotype and very high insulin requirement, will uncover this rare entity of KPD where insulin can be stopped, and the patient may have a remission from diabetes. The findings of clinical and biochemical characteristics in the present study could be used for the identification of patients with KPD in countries that lack the resources for high-quality testing.