1. Background
Noonan Syndrome (NS) is a genetic condition with an incidence of 1:1000 to 1:2500 and is equal between males and females (1-3). The majority of cases are autosomal dominant, but it is thought that up to 50% of cases are sporadic (1-3). The disorder is recognised by distinct clinical features, which include hypertelorism, low-set ears and ptosis (2-4). NS is also associated with cardiac abnormalities, such as pulmonary valve stenosis and left ventricular hypertrophy, hypogonadism, developmental delay, chest deformities and delayed puberty (1-4). NS has also been associated with increased risk of benign and malignant conditions, such as leukaemia and solid tumours, most in patients with PTPN11 mutation (1, 5-9).
Short stature is a recognised feature and seen in approximately 70 - 80% of patients with NS (1, 10). The mean adult height in patients with NS is 169.2cm and 154.4cm, for males and females, respectively (1, 2, 11).
The pathophysiology for short stature in NS remains unclear. It has been proposed that individuals with NS could be growth hormone (GH) deficient, have neurosecretory dysfunction or GH resistance (1). Noordam et al investigated seventeen patients with NS and associated short stature and noted that 94% of their patients had optimal GH levels following stimulation (12). However, when overnight GH secretion was monitored, half of the patients had low GH secretion suggesting the possibility of neurosecretory dysfunction (12). Six male patients had an unusual pattern of GH with high trough levels. The baseline GH levels were always above 2.5mU/L, but the peaks were similar to other participants in the study suggesting the possibility of GH resistance (12). Limal et al found low insulin-like growth factor (IGF-1) levels, especially those with a PTPN11 mutation (13). These results were supported by Binder et al’s study (14) who found that GH levels were higher in overnight secretion and stimulation tests in patients with PTPN11 mutations, compared to those without (14). These results also suggest that GH resistance may potentially cause short stature in patients with NS (14).
GH has been approved by the Food and Drug Administration (FDA) in NS, but has not yet been approved by the European Medicines Agency (3, 15).
2. Objectives
The aim of this study is to evaluate the effect of GH treatment on a cohort of patients with NS and short stature during the first two years of treatment.
3. Methods
Patients with NS treated with GH at a tertiary Children’s Hospital in Liverpool, United Kingdom, were identified. Data was collected retrospectively from the paper medical notes and information was transferred to an electronic database. Research and development approval was obtained (number 6094) and informed consent was not required. Information collected included length of treatment to date and height velocity (HV) for each year on medication. The height measurements were undertaken during the outpatient appointments by trained health care professionals using a standardised stadiometer.
The birth weight standard deviation score (SDS) was corrected to the gestational age. Bone age was analysed using the radius-ulna-short bones method using Tanner-Whitehouse (TW2) method. Both IGF-1 and GH are measured using solid phase, enzyme-labelled, chemiluminescent immunoassays on a Siemens Immulite 2000 XPi analyser [coefficients of variation for low and high levels were 2.82% and 3.80% respectively].
3.1. Statistical Analysis
The results were analysed with variables expressed as mean values and SDS and statistics were determined using the t-test with a P-value of less than 0.05 being classed as statistically significant.
4. Results
Fifteen patients with NS on GH therapy were identified. The full clinical details for three patients were unavailable, so twelve patients (M: F = 10:2) were included in the final analysis. The average age at the start of the treatment course was 8 years (range: 4 - 13 years). The mean birth weight was 3.23 kg (SD ± 0.69 kg; -0.17 SDS [range: -1.68 to + 0.82]).
Eight of the twelve patients had associated cardiac anomalies, with the majority being pulmonary valve stenosis (88%) and other cardiac anomalies included atrial septal defect and hypertrophic cardiomyopathy (Table 1). Baseline IGF-1 was assayed before the start of treatment in seven patients and the mean IGF-1 was 7.4nmol/L (SD ± 6.95; range: 12.5 - 67.2nmol/L). GH stimulation tests were undertaken in two patients prior to starting GH treatment and both patients had a peak GH level above 7 micrograms/L. Five patients had their bone age (BA) analysed with three results within normal limits (1. BA 4.16 years and chronological age (CA) 4.25 years; 2. BA 15.09 years and CA 15.69 years; 3. BA 4.32 years and CA 4.5 years). Two patients showed delayed BA (1. BA 11.28 years and CA 15.06 years; 2. BA 6.7 years and CA 11 years). Ten of the twelve patients were pre-pubertal prior to starting treatment.
| Patient | Co-Morbidities |
|---|---|
| 1 | PVS |
| 2 | Mild PVS |
| 3 | PVS |
| 4 | Significant scoliosis (corrected) |
| Large cervical syrinx | |
| Delayed puberty | |
| 5 | PVS |
| ASD | |
| FTT | |
| 6 | Right pelvi-ureteric junction obstruction |
| 7 | Chiari 1 malformation with cervical syrinx |
| Pectus carinatum | |
| 8 | Thrombocytopenia and neutropenia |
| PVS | |
| Asthma | |
| Orchidopexy | |
| 9 | PVS |
| ASD | |
| Orchidopexy | |
| 10 | HOCM |
| Persistent left lower lobe collapse | |
| 11 | PVS |
| 12 | Craniofaciocutaneous syndrome |
| Left sided hearing loss | |
| Nystagmus and squint | |
| Sagittal synostosis (previous operation) |
Abbreviations: PVS, Pulmonary Valve Stenosis; ASD, Atrial Septal Defect; FTT, Failure to Thrive; HOCM, Hypertrophic Cardiomyopathy.
The mean treatment duration was three years, ranging from 1 - 8 years. The average starting dose of GH treatment was 0.034 milligrams per kilogram per day (mg/kg/day). The dose was adjusted during the course according to the response and the maximum mean dose was 0.037mg/kg/day (range: 0.027 - 0.045mg/kg/day). Following treatment, the mean IGF-1 improved to 27.5nmol/L (SD ± 15.9; range: 12.5 - 44.1 nmol/L).
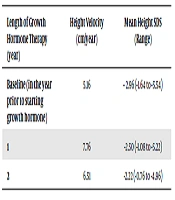
The mean baseline height SDS was -2.96, ranging from -1.64 to -5.54. After one year of treatment, the mean height SDS improved to -2.50, which was statistically significant (P = 0.0035, 95% CI -0.8037 to -0.2163). The mean height SDS continued to improve at the end of 2 years following treatment to -2.22 (P = 0.0020, 95% CI -1.1609 to -0.3916) (Table 2). The mean HV for the patients prior to starting treatment was 5.16cm/year (ranging from 2.4 to 8.2cm/year), which significantly improved to 7.76cm/year (ranging from 4.1 to 12.8cm/year) after one year of GH treatment (P = 0.02). The HV for the second year remained above baseline with an average of 6.51cm/year (P = 0.20 compared to baseline HV) (Table 2). The HV reduced at the end of three years of treatment to 4.95cm/year and continued to vary over the next five years, although the actual number of patients on treatment beyond two years were less.
| Length of Growth Hormone Therapy (year) | Height Velocity (cm/year) | Mean Height SDS (Range) |
|---|---|---|
| Baseline (in the year prior to starting growth hormone) | 5.16 | - 2.96 (-1.64 to -5.54) |
| 1 | 7.76 | -2.50 (-1.08 to -5.22) |
| 2 | 6.51 | -2.22 (-0.76 to -4.96) |
5. Discussion
The results of the study show that the height SDS in patients with NS significantly improved in the first two years of GH therapy compared to baseline. HV improved significantly during the first year of GH treatment and remained higher than baseline for the second year.
Kirk et al undertook a study in 2001 with a sample size of sixty-six patients with NS treated with GH (10). Similar to our results, the authors showed a significant improvement in HV over the first year of treatment (10). They followed the patients for up to six years and found that the growth slowed down after three years of therapy. The final height was thought to be improved in this group, but only a small number increased by over five centimetres (10). MacFarlane et al also looked at the effect of GH treatment in patients with NS compared to those not treated (4). The HV was again significant in the first year and remained high during the second year, but less significant (4). In 2015, Zavras et al compared growth between a group of NS patients with GH deficiency and a group of patients with isolated GH deficiency (15). Interestingly, the results showed that the patients with NS responded to the treatment best during the first three years, with significant improvements in their height during the second and third years (15). Overall though, the patients with NS had significantly lower heights when compared to the patients without NS over a five year period (15).
The results of our study also showed that most of the patients had low IGF-1 levels prior to starting GH treatment and that the levels increased in all these patients during treatment. Noordam et al supported this result by showing that the height and HV of patients with NS at baseline were significantly associated with their IGF-1 levels (12). GH treatment was then shown to increase IGF-1 levels, which was associated with a significant increase in HV (12).
The genotypes have previously been shown to influence the height of patients with NS. Limal et al looked at the effect of the PTPN11 mutation on height and showed that patients with a PTPN11 mutation were significantly shorter than those without at the age of six years (13). The authors also showed that the patients’ height did improve with treatment, but the PTPN11 positive patients were still significantly shorter after two years compared to the PTPN11 negative patients (13). The IGF-1 levels pre-treatment were noted to be low, especially in the PTPN11 mutation group (13). This level improved following one-year of treatment and there was no difference noted between the two groups (13). Similarly, Binder et al found that patients with PTPN11 mutations had significantly lower IGF-1 levels and the improvement in height following one-year of treatment was significantly lower when compared to PTPN11 mutation negative group (14).
Data regarding the effect of GH therapy on final adult height in patients with NS remains limited. As previously mentioned, Kirk et al found that the final height increased following treatment, but only a few patients gained more than five centimetres in height (10). In contrast, Romano et al showed an average improvement of 10.9 cm and 9.2 cm for males and females, respectively, following an average length of 5.6 years of treatment (16). Both studies used the same model to predict final adult height.
Seo et al reviewed twelve studies and concluded that GH treatment does improve height and HV (1). The authors concluded that factors such as longer course of treatment, starting treatment at a younger age and increased height at puberty were related to improved final adult height (1). These suggestions were also supported by Noonan et al following a review of fifteen studies (2). Giacomozzi et al published a systematic review on the impact of GH on the final adult height in patients with NS (3). The authors reviewed six studies and concluded that the evidence available is not sufficient to support the use of GH in the treatment of short stature in NS (3). The controlled trial used an average growth hormone dose of 0.047mg/kg/day, whereas the doses for the five uncontrolled trials ranged from 0.033 to 0.066mg/kg/day (3). The authors did not find a significant correlation between the dose of treatment and the response in height (3).
Finally, potential concerns have been raised about the safety of GH treatment in patients with NS, especially in those with cardiac anomalies. All the patients in our study tolerated the medication well and were regularly followed up by the cardiology and endocrinology teams. No specific side effects related to growth hormone treatment were noted in these patients. The reviews and studies discussed have also demonstrated GH therapy to be safe with no significant side effects (1-4, 15). GH has been thought to trigger proliferation of normal and malignant cells (9). Current literature has not found an association between GH therapy and increased risk of malignant disorders in patients who are not more likely to be predisposed to cancer from a genetic condition (9, 17). There is only limited data regarding the increased risk of cancer in patients with NS who are treated with GH. Krishna et al reported two NS patients who were diagnosed with brain tumours (18). Case one had a known PTPN11 mutation and was diagnosed with a dysembryoplastic neuroepithelial tumour fifteen months after starting GH treatment. His HV reduced at twelve months post tumour resection and GH therapy was restarted. The size of the residual tumour did not change over the next year and at the time of publishing, the patient remained on GH (18). Case two also had a known PTPN11 mutation and was diagnosed with pilocytic astrocytoma after eighteen months on GH treatment. The patient was fifteen years old at the time of diagnosis and then remained off GH permanently. One-year post resection, the tumour was stable. The authors are unable to conclude whether the tumours were present prior to GH treatment or whether they progressed following GH therapy. The authors recommend a baseline MRI scan of brain prior to starting GH treatment in patients with NS (18).
5.1. Study Limitations
Limitations of our study include small sample size and non-availability of data regarding the final height and genotype. Therefore, we were unable to comment on the long-term effect of GH therapy on final adult height in NS patients and whether the genotype has influenced the HV during the treatment course.
In conclusion, our study has shown that GH treatment significantly improves the height SDS over a course of two years. HV also significantly improved following GH treatment. Further research is required to understand the impact of GH on final adult height.