1. Background
Male-factor infertility, as one of the most common causes of infertility, is solely responsible for 10 - 30% of infertility cases and contributes to 50% of all infertile cases overall (1-4). Male infertility is a major public health concern with severe negative psychological burden, health costs, ostracism, and social discrimination in some contexts with a strong emphasis on childbearing.
While there has been extensive focus on female infertility, according to the literature, there is a correlation between some chronic disorders and male infertility, varying from cardiometabolic, autoimmune, and oncologic disorders to increased mortality rate later in life (5-9). The exact underlying pathophysiology of these associations remains unclear, although it is suggested that genetic, intrauterine environment and lifestyle factors may play a role (8, 10-12).
However, not only a few studies have focused on the association between male infertility and cardio-metabolic disorders, but also their findings are controversial. Moreover, most of those studies had some important limitations, including using the surrogate markers of infertility such as varicocele or childless situation of men or following a non-population based design, which may not reflect the characteristics of the target population, lack of appropriate adjustments for potential confounders like age and BMI, which limited the generalizability of their findings. In this respect, Eisenberg et al. (2011) evaluated the association between fatherhood and the risk of cardiovascular death in a population of US males average of 10.2 years. They reported that men with no child were at increased risk of cardiovascular mortality after the age of 50 compared to those with two or more children (13). However, as being childless is not equivalent to infertility, caution should be taken when interpreting the results of this study. In another epidemiological study, Wang et al. reported that men who were suffering from varicoceles had an increased risk of developing cardiovascular problems, diabetes, and hyperlipidemia compared to men with vasectomy (14). Another retrospective cohort study, which used national databases, reported that male infertility was associated with a higher risk of developing diabetes and ischemic heart disease in the years after an infertility evaluation compared to men receiving only fertility testing (15). However, although varicoceles may be associated with male infertility, suffering from this disorder does not necessarily specify male infertility; thus, limiting the interpretability of the findings.
2. Objectives
Regarding the inaccessibility of necessary data, following a population-based design, the current study aimed to assess the association between male infertility and cardiometabolic disturbances among participants of the Tehran lipid and glucose study.
3. Methods
The current study’s participants were chosen from the Tehran Lipid and Glucose Study (TLGS). The TLGS is a long-term, the population-based study started in 1998 intended to assess the non-communicable disease risk in a representative population of men and women living in Tehran, Iran; a total of 15005, who were older than 3 years, have been monitored in intervals of 3 years, and all of their related characteristics, examinations, and measured data have been recorded. Comprehensive details of the TLGS are published previously (16, 17).
Research Institute for Endocrine Sciences ethics committee has approved the study protocol. In addition, written informed consent was obtained from all participants.
3.1. Study Population
For the purpose of the present study, we used data collected in the third follow-up visit of TLGS, which included comprehensive data on the reproductive status of participants (16). All those who were never married, were unwilling to have a child or had documented female infertility were excluded from the study. Eventually, in total, 1611 eligible individuals were categorized into two groups of men with documented male infertility (n = 88) and those with at least one live birth and no history of primary infertility (n = 1523).
3.2. Measurements
All clinical, anthropometric, and biochemical parameters were measured by trained staffs. Comprehensive details for the TLGS measurement are published elsewhere (18, 19). In brief, blood samples were taken after 12 h of overnight fasting. Triglyceride (TG) levels were measured using glycerol phosphate. Total cholesterol (TC) was measured using the enzymatic colorimetric method with cholesterol esterase and cholesterol oxidase. High-density lipoprotein cholesterol (HDL-C) was assayed based on modified Friedewald to calculate LDL-C. All metabolic analyses were performed using (Pars Azmon Inc., Tehran, Iran) and a Selecta 2 autoanalyzer (Vital Scientific, Spankeren, Netherlands) kits. Intra/inter-assay coefficients of variations for TG, TC, HDL-C, and LDL-C were less than 2.1, 1.9, 3, and 3%, respectively. Serum creatinine (cr) concentrations were measured by kinetic colorimetric Jaffe; sensitivity of the assay was 0.2 mg/dL (range: 18 - 1330 µmol/L (0.2 - 15 mg/dL). Intra/inter-assay CVs were less than 3.1%. All biochemical assays were performed using commercial kits (Pars Azmoon Inc., Tehran, Iran) using a Selectra 2 autoanalyzer (Vital Scientific, Spankeren, The Netherlands). Assay performance was monitored after every 25 tests using lyophilized serum controls in normal and pathologic ranges. All samples were analyzed only when the internal quality control met the standard acceptable criteria.
3.3. Definition of Terms
Infertility was defined as the inability of a ‘couple’ to get pregnant despite 12 or more months of unprotected sexual intercourse (20), and male infertility was defined as any infertility that attributed to male factor diagnosed by sperm parameters below the normal values recommended by the world health organization (21). In the present study, information about male infertility were obtained by investigating the history of infertility, using a self-reported questionnaire, and were further confirmed by medical documentation. Hypertension was defined (22) as a mean systolic blood pressure ≥ 140 mmHg, mean diastolic blood pressure ≥ 90 mmHg, or undergoing treatment for hypertension. Metabolic syndrome was defined (23) as having at least three of the following five criteria: TG concentration of ≥ 150 mg/dL or receiving specific medication; HDL ≤ 40 mg/dL or receiving specific medication; SBP ≥ 130 mmHg, or DBP ≥ 85 mm Hg, or receiving specific medication; FPG ≥ 100 mg/dL or receiving specific treatment; and waist circumference ≥ 90 cm for men according to the Iranian population-specific threshold. Dyslipidemia was defined as a TG level ≥ 240 mg/dL, or LDL ≥ 160 mg/dL, or TG ≥ 200 mg/dL, or HDL < 40 mg/dL, or receiving specific medication (24). Obesity was defined as BMI ≥ 30 kg/m2, and central obesity was defined as a WC ≥ 90 cm (25). Chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate (GFR) < 60 mL/min/1.73 m2 (26). In this study, GFR was calculated using the abbreviated prediction equation, provided by the modification of diet in renal disease (MDRD) study as follows: GFR = 186 × (SCr) -1.154 × (Age) -0.203, in which eGFR (estimated GFR) is presented as mL/min per 1.73 m2, and serum creatinine (Scr) is presented as mg/dL. Diabetes was defined as FPG ≥126 mg/dL or 2-hpost-challenge plasma glucose (2 h-PCPG) ≥ 200 mmol/L or taking anti-diabetic medication in all phases of the study (27).
3.4. Statistical Analyses
Based on the primary outcome of hypertension, which refers to the probability of detecting a difference between study groups when a true difference exists (prevalence of HTN in the infertile and fertile group: 39 and 26%, k: group ratio 1523/88 ≈ 17 and type one error: 5%), the statistical power was estimated at 70%. The Kolmogorov-Smirnov test was applied to test for a normal distribution. Baseline characteristics of both groups were compared using the Mann-Whitney U, t-student, and chi-squared tests. Logistic regression was performed to investigate the association between cardiometabolic disturbances and male infertility. In addition, odds ratios with a 95% confidence interval were estimated to show the association. Moreover, penalized logistic regression via the data augmentation method was used to avoid potential biases. Statistical analyses were performed using STATA version 14 (STATA Inc., College Station, TX, USA), and the Penlogit STATA package was applied to run the data augmentation method. Statistical significance was considered when P-value < 0.05.
4. Results
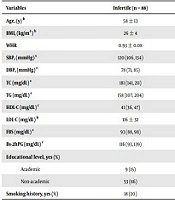
Baseline characteristics of participants are presented in Table 1. Compared to fertile controls, infertile men were more likely to be older, [58 (13.3) versus 53 (10.2) years (P = 0.003)], respectively, and had significantly higher SBP [120 (28.8) vs. 115 (18) mm Hg, P < 0.001], FBS [93 (10.3) vs. 91 (11) mg/dL, respectively; P = 0.011] and Bs-2hPG [115.5 (46) vs. 104 (45) mg/dL, respectively; P < 0.0014].
| Variables | Infertile (n = 88) | Fertile (n = 1523) | P-Value |
|---|---|---|---|
| Age, (y) a | 58 ± 13 | 53 ± 10 | 0.003 b |
| BMI, (kg/m2) a | 26 ± 4 | 26 ± 4 | 0.513 |
| WHR | 0.93 ± 0.08 | 0.93 ± 0.08 | 0.789 |
| SBP, (mmHg) c | 120 (106, 134) | 115 (106, 124) | 0.001 b |
| DBP, (mmHg) c | 78 (71, 85) | 78 (71, 85) | 0.155 |
| TC (mg/dL) c | 181 (141, 211) | 191 (167, 215) | 0.307 |
| TG (mg/dL) c | 158 (107, 204) | 161 (107, 216) | 0.841 |
| HDL-C (mg/dL) c | 41 (36, 47) | 42 (36, 48) | 0.883 |
| LDL-C (mg/dL) a | 116 ± 32 | 117 ± 32 | 0.640 |
| FBS (mg/dL) c | 93 (88, 98) | 91 (85, 97) | 0.011 b |
| Bs-2hPG (mg/dL) c | 116 (93, 139) | 104 (81, 127) | 0.014 b |
| Educational level, yes (%) | 0.584 | ||
| Academic | 9 (15) | 171 (14) | |
| Non-academic | 53 (86) | 1037 (86) | |
| Smoking history, yes (%) | 18 (20) | 257 (21) | 0.539 |
Abbreviations: BMI, body mass index; WHR, waist to hip ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein; LDL-C, low-density lipoprotein cholesterol; FBS, fasting plasma glucose; Bs-2hPG, 2-hour plasma glucose.
a Values are expressed as mean ± SD.
b Values are statistically significant.
c Values are expressed as median (Q1, Q3).
The crude age and BMI adjusted associations between cardio-metabolic disorders and male fertility status are provided in Table 2. The unadjusted model revealed a significant association between infertility and hypertension and CKD (OR = 1.8; 95% CI: 1.2, 2.9; P = 0.006, and OR = 1.9; 95% CI: 1.1, 3.6; P = 0.033, respectively). However, the significant association found in the crude analysis was disappeared after adjusting for potential confounders of age and BMI. Moreover, infertility did not have any association with other cardiometabolic disturbances, including diabetes and pre-diabetes, metabolic syndrome, dyslipidemia, obesity, and central obesity in both unadjusted and adjusted analyses.
| Outcomes | Infertile (n = 88) | fertile (n = 1523) | Unadjusted OR (95% CI) | Age-BMI adjusted OR (95% CI) |
|---|---|---|---|---|
| Diabetes mellitus | 21 (32) | 300 (25) | 1.3 (0.8, 2.3) | 1.1 (0.6, 2.0) |
| Pre-diabetes | 26 (30) | 368 (24) | 1.3 (0.8, 2.1) | 1.1 (0.7, 1.8) |
| Hypertension | 34 (39) | 389 (26) | 1.8 (1.2, 2.9) b | 1.4 (0.8, 2.3) |
| Metabolic syndrome | 48 (55) | 731 (48) | 1.3 (0.8, 2.0) | 1.1 (0.7, 1.9) |
| Dyslipidemia | 47 (53) | 822 (54) | 0.9 (0.6, 1.5) | 1.0 (0.6, 1.7) |
| Obesity | 23 (26) | 366 (24) | 1.1 (0.7, 1.9) | 1.5 (0.4, 5.7) |
| Central Obesity | 32 (36) | 503 (33) | 1.1 (0.7, 1.9) | 1.1 (0.6, 2.1) |
| CKD | 13 (15) | 125 (8) | 1.9 (1.1, 3.6) b | 1.3 (0.6, 3.5) |
a Values are expressed as No. (%) unless otherwise indicated.
b Value is statistically significant (P-value < 0.05).
5. Discussion
In the present study, following a population-based cross-sectional design, we found no association between the history of male infertility and cardio-metabolic disturbances in infertile men compared to their healthy fertile counterparts. Male infertility is a multi-dimensional problem that is expected to grow during the next two decades (28). Emerging evidence suggests an intertwined link between male infertility and their overall health status (6, 29). However, in the last two decades, several studies have investigated the prevalence and incidence of some cardio-metabolic comorbidities in populations of patients with male infertility. In contrast to our findings, most data from these series supported the association between infertility and those disturbances (6, 30-33). In this respect, Lawlor et al. (2003) assessed the association between the prevalence of coronary heart diseases (CHD) and the number of offspring in a sample of the British population. They showed that men with ≤ 1 child had a higher risk of CHD compared to those with more children (34). In the same vein, Ringbäck Weitoft analyzed cause-specific mortality data of 682919 lone fathers and childless men living in Sweden and reported an enhanced risk of ischemic heart disease (35). Likewise, Eisenberg et al. investigated the association between semen secretion and medical comorbidity in a cohort of 9387 men with available semen analysis in a fertility clinic. They reported a significant association between cardiovascular disease (i.e., hypertension, peripheral vascular diseases, cerebrovascular diseases, and non-ischemic heart diseases) and a significantly higher rate of any type of semen abnormality (30). In another recently published study, Helene Glazer et al., in a Danish national IVF register-based cohort study on 39516 men with a history of fertility treatment, reported that male infertility may contribute to the development of diabetes mellitus. However, those risks were related to the severity of the underlying fertility factors (36). The results of our population-based study are not in line with the literature, which supports the association between cardio-metabolic disturbances and male infertility. These disparate findings can be attributed to differences in methodologies applied to measure fertility. Unlike female infertility, treating male infertility is a challenging issue and is not well investigated in general (1), which may potentially lead to conflicting results. In this respect, male infertility has not been defined as an independent disease (1). In the lack of a unique definition, most studies have used various criteria like infertility-associated disorders or the childless situation of men. However, although those may contribute to male infertility, but do not necessarily imply male infertility; thus, limiting the interpretability of the data. Moreover, most studies on male infertility did not have a population-based design and were performed in the tertiary settings, mainly infertility clinics, which potentially include severe forms of infertility that are not a representative sample of the larger population of infertile men, which may indicate an important bias. In addition, in some contexts, men disclaim infertility help-seeking traditionally and do not usually agree to undergo fertility evaluation, resulting in underestimating male infertility. However, different populations vary by age range, ethnicity, and unit of measurement, as well as other risk factors, which probably have affected the results.
It is necessary to mention some limitations and biases of our study, including only evaluating Iranian men, which limited the generalizability of the findings to other contexts.
Furthermore, infertility diagnosis was self-reported, which may be limited by recall bias. However, the diagnoses were further confirmed by reviewing the medical records of participants. In addition, previous studies found a negligible association between self-reported and confirmed infertility (37, 38). Moreover, since the infertility data were collected in the third phase of the TLGS, we could not perform a longitudinal study to assess the risk of cardiometabolic events in infertile men due to insufficiency of infertile individuals and short-term follow-up. However, since TLGS is an ongoing study, it will let us perform such analyses with a longitudinal design in the future.
However, since a cross-sectional design was followed, we did not identify the causality between infertility and cardiometabolic disturbances. Long-term prospective studies are needed to investigate those causality effects.
5.1. Conclusions
In conclusion, our study demonstrated no association between male infertility and cardiometabolic disturbances. By focusing solely on men in a population-based setting, we tried to fill the gaps in knowledge in the infertile male population. The findings can pave the way for further studies to extend our knowledge in this field. More population-based studies with a large sample size are warranted to confirm these findings.