1. Context
Enhanced public health, along with improved socioeconomic conditions over the past few decades, has led to the control of various contagious and lethal diseases. However, the extensive revolution of the human lifestyle, especially in terms of physical activity and eating habits, has increased the prevalence of chronic and non-communicable diseases, such as diabetes. Obviously, the cornerstone for the prevention and management of these problems would be lifestyle modification. Exercise and exercise therapy have always been an integral part of diabetes management and prevention, and their importance has increased each and every day. Therefore, a part of each authentic diabetes clinical guideline is allocated to exercise and physical activity. However, there is no clinical practice guideline that specifically focuses on exercise in Iranian patients with diabetes, except for a few review articles and consensus statements (1, 2). This was an attempt to bridge this gap by collecting and evaluating the existing scientific documents in a coherent manner, specifically for answering questions concerning exercise in diabetes.
1.1. Epidemiology
According to the most recent Atlas of the IDF 2019, it is estimated that about 463 million adults have diabetes, and this will reach 700 million by 2045. This number was about 151 million in 2000 and has grown more than three times during this period. The worldwide prevalence of diabetes has also risen from 4.7% in 1980 to 9.2% in 2019 (3). During the last decade, the rate of increase in the prevalence of diabetes in low- and middle-income countries has been higher than in rich countries (4). According to a 2016 report by the World Health Organization (WHO), the prevalence of diabetes in Iran was 10.3% (5). However, the crude prevalence of prediabetes and diabetes among the adult population of Tehran may have been up to 26.43 and 16.27%, respectively (6). Type 2 and type 1 diabetes comprise 85.5 and 11.4% of the registered Iranian population with diabetes, respectively, and the remaining suffer from the other types of the disease (7). In Iran, about 5% of pregnant women develop gestational diabetes (8).
Considering the benefits of regular exercise in lowering diabetes incidence, blood glucose levels, and diabetes risk factors such as for overweight and obesity, decreasing physical inactivity by 10% has been targeted worldwide (4). However, the sedentary lifestyle has become a global concern, with a quarter of the adult population not doing the minimum recommended amount of physical activity. A sedentary lifestyle is more common in women around the world (27% of women versus 20% of men), and has become alarmingly common among adolescents (84% in girls and 78% in boys) (4). The physical activity profile of people living in Middle Eastern countries is low compared to other countries. In detail, the average amounts of global physical activity for women and men are around 3000 and 3500 MET-minutes per week, respectively, albeit these values are less than 2000 for women and 2500 for men in these countries (9). Other evidence testifies that the prevalence of a sedentary lifestyle among the Iranian population is more than its worldwide average and reaches between 30 and 70%. Lack of physical activity is more common in the southeast and near the center of the country (10).
Although overweight and obesity are strongly associated with diabetes, in 2014 one-third of the world population were overweight and 10% were suffering from obesity. Overweight is more common in women than in men (4). In Iran, obesity is also more prevalent among women than men (38.1 vs. 23.4%), although the waist circumference of middle eastern men is higher than women’s waist circumference (9).
1.2. Objectives
This document aims to provide well-defined, simple, and concise responses to certain questions related to physical activity and exercise in all patients with diabetes, including type 1, 2, and gestational diabetes mellitus (GDM). We also highlight the clinical significance of physical activity and exercise and give priority to the subjects of them.
1.2.1. General Objective
To gather the best available scientific evidence as a comprehensive guideline for clinical practice and health care. This guideline supports the efficient and rational use of exercise and physical activity in the prevention and management of diabetes.
1.2.2. Specific Objectives
- To establish the value of regular exercise as a lifestyle strategy in the prevention and management of different types of diabetes.
- To specify the appropriate exercise prescription in a multidisciplinary approach.
- To define special considerations and precautions for exercise in patients with or without diabetes-related complications.
2. Evidence Acquisition
2.1. Target Population
The recommendations presented here are targeted to all individuals at risk or involved with any type of diabetes mellitus.
2.2. Target Users
Given the burden and importance of diabetes, the recommendations of this clinical practice guideline should be targeted to all healthcare personnel responsible for different levels of clinical care of patients with diabetes.
This group includes: (1) all medical staff who are responsible for screening, initial management, or referral of patients with diabetes including general practitioners and internists; (2) endocrinologists and physicians who have received specialized formal training in diabetes management; (3) sports and exercise medicine specialists and physicians who have received formal training in exercise prescription for patients with diabetes; (4) healthcare workers who give education and services to patients in primary care clinics for diabetes; (5) specialists in general and clinical nutrition; (6) patients at risk for or involved with diabetes.
2.3. Methods
A multidisciplinary team of experts, consisting of sports medicine specialists, endocrinologists, and cardiologists participated in developing this clinical practice guideline. All participants in the panel declared no conflicts of interest. This group did the task in four stages:
- Identifying and refining the subject area as 17 clinical questions using population, intervention, control, and outcomes (PICO) criteria (11).
- Appraising evidence which was reaped during a systematic review of the literature. This review assured a systematic search of the scientific evidence (including practice guidelines and systematic reviews of the literature as primary sources). To assess the quality and address the variability of the existing guidelines, the group used the appraisal of guidelines for research & evaluation (AGREE) instrument (12).
- Extracting recommendations from evidence.
For grading the recommendations, we used the systematic method of the Oxford Centre for Evidence-Based Medicine (OCEBM) to determine how well the evidence answers the clinical question of interest. The recommendations were graded as A, B, C, D, or I based on the quality, quantity, and consistency of existing evidence. When the recommendation was obtained from Level 1 evidence with consistent results, it was given a grade A. In this case, a strong recommendation can be made for or against a particular piece of advice. Grade B recommendations signify a fair level of confidence for making a recommendation. This grade is based on consistent level 2 or 3 studies or extrapolations from level 1 studies that show promise. Grade C recommendations are based on evidence that is conflicting or primarily based on level 4 studies or inferred from level 2 or 3 studies. It is hard to make a recommendation for or opposed to a specific piece of advice when the source of evidence only allows a grade C. A grade D implies that insufficient evidence to compose a recommendation. This grading system indicates how confident clinicians can be when making a clinical application of research findings (13).
Initial recommendations were assessed and scored by the expert panel group, checking for their clinical advantage and adaptability. Finally, recommendations with high scores of appropriateness and agreement were selected.
4. Subjecting the guideline to the external review. The final version of the guideline was evaluated and approved by the National Deputy for Curative Affairs - Ministry of Health and Medical Education. The guideline was also endorsed by the Iran Endocrine Society (IES) and the Iranian Association of Sports and Exercise Medicine (IASEM).
3. Results
3.1. Role of Physical Activity in the Prevention and Management of Diabetes
3.1.1. Physical Activity and Diabetes Risk Reduction
Question 1: Is physical activity effective in diabetes prevention?
To investigate the effect of physical activity on diabetes prevention, a few systematic reviews have been conducted. A recent systematic review by Sheng et al. showed that in adults with pre-diabetes, lifestyle modifications, involving nutrition, exercise, and weight loss, reduce the incidence of diabetes and enhance the health status by improving body composition indices and lipid profile, in terms of total cholesterol and HDL, and decreasing both systolic and diastolic blood pressure (14). According to another systematic review and meta-analysis by Schellenberg et al., moderate evidence exists that a comprehensive lifestyle intervention including exercise diminished the risk for type 2 diabetes in high-risk persons (15). Similarly, Aguiar et al. presented in a systematic review that multi-component lifestyle interventions, including diet along with aerobic and resistance exercise, have modest useful effects on body weight, fasting blood glucose, and glucose tolerance, and induce dietary and exercise outcomes in patients with prediabetes or at-risk adults (16). To prevent and treat chronic metabolic diseases, including diabetes, American Diabetes Association (ADA) generally recommends a minimum of 150 minutes of moderate to vigorous aerobic exercise each week (17).
Regarding the effect of exercise in the prevention of gestational diabetes (GDM), the results of most systematic reviews are promising. For example, Yu et al. indicated in a systematic review that exercise intervention could significantly reduce the risk of GDM, but they did not reveal any prominent influence on gestational age at birth, preterm labor, 2-hour glucose tolerance test, birth weight, or low Apgar score (18). Also, a metanalysis by Russo et al. showed that physical activity during pregnancy introduces slight protection against developing GDM with a 28% risk reduction (19). Almost similar results were reported in two other systematic reviews addressing normal weight and overweight or obese pregnant women, respectively (20, 21). In addition, another systematic review by Yin et al. found insufficient evidence to support the preventive effect of increased physical activity (22). Generally, physical activity is a valuable measure to prevent diabetes in all at-risk individuals (14-33).
Recommendation 1: Lifestyle modification focusing on adequate physical activity, proper nutritional diet, and weight reduction should be recommended to all persons at high risk for diabetes (level A).
Recommendation 2: To prevent the occurrence of type 2 diabetes, at-risk adults should perform at least 150 minutes per week of moderate to severe physical activity as a part of lifestyle modification (level A).
Recommendation 3: Regular exercise should be recommended to all pregnant women, regardless of body weight, to reduce the risk of gestational diabetes without jeopardizing birth outcomes (level B).
3.1.2. Physical Activity and Diabetes Management
Question 2: Is physical activity effective in diabetes management?
Both regular physical activity and organized exercise, in people with type 2 diabetes, introduce some adaptations in the liver and muscular and adipose tissues, which lead to the enhancement of insulin function, even deprived of weight loss (34-36). The most obvious improvements occur in patients with greater insulin resistance in the baseline (37). Both aerobic and resistance training may exert these positive effects. However, they have a greater influence on glycemic control when prescribed in combination than in programs that just include one type of exercise. Furthermore, patients may benefit from positive exercise-related changes in other cardiovascular risk factors (38).
A meta-analysis in patients with type 1 diabetes did not show any evidence for positive effects of exercise on glycemic control by means of HbA1c. Extra calorie consumption and decreasing insulin dosage around the training times, or low power of studies are some of the possible reasons for this finding (39, 40). Nevertheless, a randomized crossover trial showed that adults with type 1 diabetes may yield some improvement in their glycemic control by performing resistance training, even with making adjustments in insulin dosage and calorie intake. However, exercise has other proven benefits in type 1 diabetes and is a vital component for its control (39).
A systematic review by Harrison et al. showed that a minimum of three times per week moderate-intensity aerobic or resistance exercise improves postprandial glucose levels and other glycemic indexes in women diagnosed with gestational diabetes (41). As conclusion, physical activity plays a critical role in the management of all patients with diabetes (23-25, 27-29, 31-46).
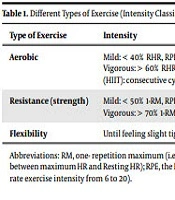
Recommendation 4: To achieve optimal blood glucose levels, patients with type 2 diabetes should perform a minimum of 150 minutes per week aerobic activity with a moderate intensity such as brisk walking along with resistance training and flexibility exercise or its equivalent (level B) (Table 1).
| Type of Exercise | Intensity | Examples |
|---|---|---|
| Aerobic | Mild: < 40% RHR, RPE: 6 - 11; Moderate: 40 - 60% RHR, RPE:12 - 13; Vigorous: > 60% RHR, RPE: 14 - 20; High intensity interval training (HIIT): consecutive cycles of moderate and vigorous intensity. | Walking, jogging, running (ground or treadmill), cycling, swimming, aqua-aerobic, rowing, in-line skating, cross-country skiing, exercising on stair-climber or elliptical machine. |
| Resistance (strength) | Mild: < 50% 1-RM, RPE: 6 - 11; Moderate: 50 - 70% 1-RM, RPE: 12 - 13; Vigorous: > 70% 1-RM, RPE: 14 – 20. | Free weights (dumbbell, barbell or kettlebells), weight machines, resistance bands, exercise balls. |
| Flexibility | Until feeling slight tightness or inconvenience. | Stretching (static and ballistic), yoga. |
Different Types of Exercise (Intensity Classifications and Practical Examples) (47)
Recommendation 5: Patients with type 1 diabetes should be encouraged to participate in physical activity and organized sports with the aim of reducing the risk of cardiovascular diseases (level B).
Recommendation 6: In patients with GDM, doing physical activity can decrease the mother’s blood glucose (level B).
3.2. Pre-participation Evaluation and Exercise Prescription in Patients with Diabetes
3.2.1. Pre-participation Medical Evaluation in Patients with Diabetes
Question 3: How should the patients with diabetes be medically evaluated before starting the exercise program?
Diabetic patients are potentially susceptible to some health risks such as cardiovascular events, hypoglycemia, or hyperglycemia by doing physical activity (32). Therefore, some authorities such as the American College of Sports Medicine (ACSM) recommend that prior medical clearance from a health care professional is necessary for any current sedentary person with diabetes who is going to get physically more active at any intensity (even low intensity) (48). According to the position statement of the ADA, this recommendation is too conservative. The available evidence does not support any screening protocol with the ability to lower the risk of critical events in asymptomatic patients with diabetes more than usual diabetes care. On the other hand, the risk is low in those who are intending to engage in low to moderate-intensity physical activities (48, 49). Therefore, diabetic individuals with no symptoms do not need any further investigations if they are receiving their routine diabetes care and wish to start with low or moderate-intensity activities. However, it is rational to take a more profound checkup and a possible exercise stress test for those who desire to raise the intensity of their activities or have a high-risk profile (32). Therefore, decision making regarding pre-participation medical evaluation should be individualized based on the symptoms, signs, care standards, and desired intensity of physical activity (25-27, 32, 48-52).
Recommendation 7: In asymptomatic patients with diabetes who plan to do low to moderate physical activity (brisk walking and activities of daily living), medical evaluation and physician clearance are not needed before starting the activity (level B).
Recommendation 8: In patients with diabetes with at least one of the diabetes complications who aim to do physical activities with intensities more than brisk walking, medical evaluation is necessary. This should comprise history taking, medical examination (including fundoscopy, foot examination, and neuropathy screening), resting ECG, and exercise stress testing (if indicated) (level D).
Recommendation 9: Cardiovascular screening using exercise stress testing is recommended for patients with a history of low physical activity plus cardiovascular risk factors, elderlies (more than 65 y), high risk for cardiovascular diseases, and patients with diabetes complications, especially in cases who plan to do physical activities more than walking (level C).
3.2.2. Recommended Types of Exercise for Patients with Diabetes
Question 4: What types of exercise are suitable for patients with diabetes?
Regarding the best exercise modalities for patients with type 2 diabetes, Pen et al. showed in a recent systematic review and network meta-analysis that not only aerobic exercise but also resistance training is helpful in improving HbA1c, although they are more effective when performed in combination. Aerobic exercise is also associated with significant improvement in fasting glucose, triglyceride, total cholesterol, and LDL plasma levels. In addition, systolic blood pressure and total cholesterol are positively influenced by resistance exercise (53).
A systematic review by Yardley et al. indicated that aerobic training improves cardiorespiratory fitness and reduces the needed insulin dose in adults with type 1 diabetes. Now, there are inadequate high-quality studies to establish the real impact of exercise training on HbA1c in patients with T1D, but recent results are encouraging (54).
A systematic review and meta-analysis by Yang et al. compared resistance and aerobic exercises for type 2 diabetes. The authors deduced that the measures of diabetes control and physical fitness are not clinically different between the two groups. Existing evidence has not shown a difference between resistance and aerobic exercise in effect on cardiovascular risk profile or safety (55).
Regarding flexibility and balance training in patients with diabetes it has been shown that stretching improves the range of motion around joints and flexibility, although without effects on glycemic control (56). The risk of falling may be reduced by balance training due to improved balance and gait, even when peripheral neuropathy is present (57). The advantages of other training modalities such as yoga and Tai Chi are less ascertained although they can bring some benefits (58, 59). As conclusion, a program including aerobic, resistance, flexibility, and balance exercises should be tailored for patients with diabetes (24, 26-29, 45, 52-65).
Recommendation 10: Regular aerobic exercise is recommended for individuals with type 2 diabetes to enhance glycemic control and decrease the cardiovascular risk factors (level A).
Recommendation 11: Regular aerobic exercise is recommended in patients with type 1 diabetes for the reduction of cardiovascular disease risk factors, although it may not be effective for glycemic control (level B).
Recommendation 12: Patients with types 1 and 2 diabetes are encouraged to do resistance (strength) training, except when there is a contraindication. Combined aerobic and resistance training is probably more effective than each modality alone (level B).
Recommendation 13: Flexibility and balance exercises and related sports such as yoga and Tai Chi may be useful when added to aerobic and resistance exercises, but they should not replace them (level C).
3.2.3. Optimal Exercise Program for Patients with Diabetes
Question 5: What is the ideal exercise prescription for patients with diabetes?
Exercise prescription for patients with diabetes should include detailed description of the four main components of mode, frequency, duration, and intensity. The prescription should be individualized based on comorbidities, contraindications, and practical goals (66).
However, in sedentary patients, low-intensity aerobic training as little as 400 kcal/week can also improve insulin sensitivity, but this dose-response effect can be fortified up to 2500 kcal (37).
Published guidelines for patients with diabetes suggest a weekly program of at least 150 minutes of continuous aerobic exercise with moderate to vigorous intensity distributed over a minimum of three days per week. However, an interesting study that evaluated the effect of exercise on all-cause mortality and cardiovascular diseases among weekend warriors proved this effect (67). Nonetheless, different studies have suggested that doing exercise regularly with higher frequency is more effective in reducing HbA1c level, and a dose-response inverse relationship has been reported between self-reported bouts of physical activity per week and glycemic control and diabetes-related complications (68). Guidelines also recommend resistance exercise, at least two days per week. Flexibility and balance exercises are also advised. Most guidelines recommend the combination of aerobic and resistance exercise within the same session (66).
Low-volume high-intensity interval training (HIIT), which involves alternating short periods of very intense anaerobic exercise with longer recovery periods at low to moderate intensity, is a substitute for continuous aerobic training (69). Nevertheless, its efficacy and safety remain unclear for some patients with diabetes (70). Special consideration should be made for properly designing different components of the exercise program (type, duration, frequency, and intensity) for all types of diabetes and age groups (19, 24, 26, 27, 29, 30, 32, 33, 43, 46, 56, 57, 60-62, 64, 66-77).
Recommendation 14: Adults with types 1 and 2 diabetes should restrict the daily sedentary time and do moderate to vigorous-intensity aerobic exercise [50 - 80% reserve heart rate (RHR)] at least 150 min/week. This time should be distributed in three or more days of a week, in such a way that the patient does not remain inactive for more than two consecutive days (level A for type 2 diabetes and B for type 1). Patients with a low level of fitness should start with the intensity that is comfortable to do and increase the intensity with improved tolerance (level D). Younger patients with a higher level of fitness can replace 150 min/week of moderate-intensity aerobic exercise with 75 min/week of vigorous-intensity aerobic (equal or more than 80% of RHR) and/or interval exercises, provided there is no contraindication (level B for type 2 diabetes and C for type 1).
Recommendation 15: Adults with diabetes should perform resistance exercises on 2 - 3 nonconsecutive days per week. The intensity of 50 - 80% one-repetition maximum (1-RM) is recommended, depending on the level of fitness. In each session, 5 - 10 exercises, involving big muscles of the upper and lower extremities and the trunk should be performed in set (s) with 10 - 15 repetitions. Each exercise should be repeated 1 and maximum of 3 - 4 sets in each session (level B).
Recommendation 16: Flexibility and balance exercises are advised 2 - 3 times/week or more. In flexibility exercises, the stretch should be maintained for 10 - 30 seconds and repeated 2 - 4 times. Yoga and Tai Chi can be used to improve flexibility, balance, and muscle strength (level C).
Recommendation 17: Children and adolescents with type 1 or 2 diabetes or at-risk persons should do at least 60 minutes of daily aerobic exercise with moderate to vigorous intensity. Muscle and bone-strengthening activities should also be performed at least three days per week (level C).
Recommendation 18: It is recommended for pregnant women with diabetes or at risk of it to perform moderate-intensity exercise for 20 - 30 min/day on most or all days of the week unless contraindicated. Sedentary women should begin with as little as 10 minutes a day and add the duration each week until they can stay active for 30 minutes a day (level B).
3.3. Blood Glucose Measurements and Preventive Interventions Against Hypoglycemia/Hyperglycemia in Patients with Diabetes
3.3.1. Pre-exercise Measurement of Blood Glucose for Patients with Diabetes
Question 6: Is blood glucose measurement recommended before exercise in patients with diabetes?
Exercise-induced hypoglycemia is a common concern for patients with diabetes and their exercise caregivers. ADA recommends pre-exercise blood glucose measurement for patients with type 1 diabetes and offers an ideal range of 90 - 250 mg/dL (32). There are some debates about the usefulness of self-blood glucose measurement (SBGM) in type 2 diabetes patients who do not use insulin, and it may improve the awareness of these patients (76). In general, pre-exercise measurement of blood glucose is beneficial (29, 32, 43, 51, 75-78).
Recommendation 19: It is recommended for patients on intensive insulin therapy (multiple daily doses, use of insulin pump) to do self-monitoring of blood glucose (SMBG) before meals and snacks, at bedtime, sometimes after a meal, before exercise, when feeling hypoglycemic, during hypoglycemia treatment until becoming normoglycemic and before performing important activities such as driving (level B).
Recommendation 20: All children and adolescents with type 1 diabetes should perform SMBG several times a day, including before meals, at bedtime, and if necessary, for increasing safety, in special circumstances, before doing sports, or with signs of hypoglycemia (level B).
Recommendation 21: Self-monitoring of blood glucose is recommended in some patients with type 2 diabetes whose treatment involves lifestyle changes or metformin to evaluate the effect of certain foods or exercise on blood glucose levels, as it can be a motivating factor in changing lifestyle. Patients with type 2 diabetes who receive basal and pre-prandial insulin are advised to undergo SMBG 2 hours post-prandial or under specific conditions to assess the effect of food, exercise, or stress on blood glucose levels (level C).
3.3.2. Blood Glucose Measurement During and After Exercise in Individuals with Type 1 Diabetes
Question 7: Is blood glucose measurement recommended during and after exercise in patients with diabetes?
Patients with type 1 diabetes show variable glycemic responses to exercise, especially when they do it non-fasted (79). An exercise program can be more finely tuned by measuring the glycemic response during and after exercise sessions (80). It seems, there are still some problems with using continuous glucose monitoring (CGM) devices during exercise, therefore, finger-stick glucose testing is the more reliable method by now (32).
In these patients, nocturnal hypoglycemia is more commonly observed at nights followed by an exercise session, than nights after a sedentary afternoon (81). Although post-exercise hypoglycemic events may occur even until 48 hours after an exercise session, typically they happen within 5-16 hours (32). As conclusion, blood glucose measurement during and after exercise may be helpful, especially in patients with type 1 diabetes (24, 29, 32, 43, 44, 79-84).
Recommendation 22: In patients with type 1 diabetes, multiple checks of blood glucose is needed to adjust insulin dose and amount of ingested carbohydrate (level B).
Recommendation 23: Patients with type 1 diabetes should be educated regarding the acute and chronic effects of exercise on blood glucose and how to adjust their insulin doses and food intake to maintain blood glucose and prevent significant hypo/hyperglycemia before, during, and after exercise (level D).
3.3.3. Optimal Glucose Level During Exercise in Patients with Diabetes
Question 8: What is the ideal range of blood glucose during exercise in patients with diabetes?
People who use insulin are suggested to consume carbohydrates before exercise if their pre-exercise glucose levels are lower than 90 mg/dL, and check for ketones if the glucose levels are greater than 250 mg/dL (32, 85). According to the latest statement of the ADA, the range between these two values is considered the ideal range of pre-exercise blood glucose (32). As a rule, exercise should be started when blood glucose is in a safe range (25, 26, 32, 43, 51, 77, 85-87).
Recommendation 24: The optimal range of blood glucose before exercise is between 90 and 250 mg/dL. In order to prevent hypoglycemia during exercise in patients receiving insulin, excess carbohydrate intake, reduced insulin dose, and selection of proper injection site are recommended (level C).
Recommendation 25: Children and young people with type 1 diabetes are advised to use carbohydrate sources if their blood glucose levels are less than 126 mg/dL before exercise (level C).
3.3.4. Measures to Reduce the Risk of Exercise-Related Hypoglycemia in Patients with Diabetes
Question 9: How to reduce the risk of exercise-related hypoglycemia in patients with diabetes?
Insulin levels do not naturally decrease with the onset of physical activity in people with diabetes who take insulin, and this can lead to hypoglycemia. Exercising in the fasted state, adjusting insulin dosage, and consuming carbohydrates if needed are strategies that have been proposed to minimize the occurrence of hypoglycemia (85). It is recommended that patients who use insulin have easy access to carbohydrate sources during exercise and ensure the safe level of their blood glucose before bedtime (80).
A systematic review that compared the effects of different modes of exercise on blood glucose level suggests that high-intensity intermittent exercise might be safer than a prolonged steady-state exercise in people with type 1 diabetes (88). A short, few seconds, sprint after an exercise bout may somehow prevent post-exercise hypoglycemia (80). Proper measures should be taken to avoid hypoglycemia during exercise (26, 32, 50, 52, 80, 85, 88-91).
Recommendation 26: To prevent hypoglycemia in patients with diabetes type 2, it is recommended to adjust the timing of medication, food, and physical activity (level C).
Recommendation 27: To maintain optimal blood glucose throughout and after exercise in patients with type 1 diabetes, increase in carbohydrate intake and/or decrease of insulin dose are usually needed (level C).
Recommendation 28: Hypoglycemia is unlikely to occur in patients with diabetes who do not use insulin, but in patients using insulin, carbohydrate intake is recommended to prevent hypoglycemia during and after exercise (level C).
Recommendation 29: Apart from insulin, some drugs (such as sulfonylureas) may increase the risk of hypoglycemia and their doses should be adjusted (level C).
3.3.5. Measures to Reduce the Risk of Exercise-Related Hyperglycemia in Patients with Diabetes
Question 10: How to reduce the risk of exercise-related hyperglycemia in patients with diabetes?
Relative insulin deficiency to compensate for the effect of increased levels of catecholamines in response to intense exercise can lead to hyperglycemia (85, 92). A rapid increase in insulin level can improve the condition, however, there is not still enough evidence for estimating an appropriate corrective dosage (85). The risk of hyperglycemia is modified by placing intense training bouts between moderate-intensity aerobic exercises (32). Individuals with type 1 diabetes whose blood glucose levels are ≥ 250 mg/dL should postpone their exercise if their blood ketone levels are increased (32, 85). In conclusion, some measures may prevent hyperglycemia during exercise (26, 32, 85, 89, 92, 93).
Recommendation 30: Due to increased postprandial blood glucose, physical activity is recommended one hour after meals. In type 2 patients with diabetes who have a blood glucose level above 300 and are not ketotic, it is advisable to exercise with caution toward general condition and hydration level (level C).
Recommendation 31: The risk of post-exercise hyperglycemia is higher in patients with type 1 diabetes, but it can be managed by insulin adjustment or cool down exercise with low-intensity aerobic exercise. Exercise in conditions of high blood glucose (> 350 mg/dL) and high blood ketones is not recommended (level C).
3.4. Environmental Factors and Exercise Limitation in Patients with Diabetes
Question 11: Do environmental conditions such as heat and cold limit exercise in patients with diabetes?
Epidemiological findings show that with the onset of heatwaves, patients with diabetes are more prone to heat-related disease and death (94). Osmotic diuresis caused by chronic hyperglycemia leads to dehydration (32). On the other hand, diabetic patients have a relative reduction in sweating, especially in the lower body, which is related to the duration of the disease, poor glycemic control, and low aerobic fitness (94). These can make the body's cooling mechanism due to perspiration inefficient. Obesity, a common co-morbidity with diabetes, is another risk for heat-related injury (95). However, heat acclimation strategies may improve whole-body heat loss in diabetes (96).
There is limited evidence suggesting that patients with diabetes are more vulnerable to cold stress (94). Paying special attention to environmental conditions during exercise is necessary for patients with diabetes (32, 91, 94-99).
Recommendation 32: To prevent heat-induced disorders, it is recommended to elderly patients with diabetes or individuals with cardiovascular, pulmonary, or neurologic complications not to exercise in places without proper air conditioning throughout hot (more than 35°C) and humid days. Obese patients should be more cautious during exercise in warm and humid conditions (level C).
Recommendation 33: Due to the reduced evaporation of skin moisture in high-humidity environments, and consequently, reduced perspiration effect on body cooling, heat-sensitive individuals such as elderlies with diabetes and patients with autonomic neuropathy and cardiovascular complications should avoid exercising in a non-ventilated environment on hot and humid days (level C).
Recommendation 34: There is insufficient evidence as to the effect of cold on exercise in patients with diabetes. However, it is recommended that patients with peripheral neuropathy, autonomic neuropathy, or peripheral vascular involvement avoid exercise in cold and frost conditions (level D).
3.5. Supervised vs. Non-supervised Exercise for Patients with Diabetes
Question 12: Is supervised exercise preferred in patients with diabetes?
Supervised training sessions may result in optimal results (38). The ADA recommends supervised exercise to yield more health outcomes from physical activity. To benefit from a safe and efficient exercise prescription, most adults with diabetes may consult with an expert exercise physiologist or fitness professional (32). A systematic review and meta-analysis, which compared the effects of supervised structural exercise with physical activity advice only, concluded that the former has a greater impact on glycemic control of type 2 diabetic patients (72). Another more recent systematic review by Pan et al. showed that supervised aerobic exercise was more effective than unsupervised aerobic and unsupervised resistance exercises in HbA1c improvement and weight control (53). Generally, supervised exercise may be more suitable, especially in high-risk patients (26, 27, 30, 32, 33, 38, 42, 53, 60, 65, 72, 74, 100, 101).
Recommendation 35: It is recommended to all patients with diabetes receive starting exercise prescription and periodic supervision by a sports medicine specialist or physician expert in the exercise prescription (level C).
Recommendation 36: Supervised exercise programs should be considered for patients with diabetes whenever possible (level B).
Recommendation 37: Patients with type 2 diabetes together with angina pectoris who are classified as high- and moderate-risk groups should perform exercise as part of a cardiac rehabilitation program (level C).
3.6. Exercise Prescription for Patients with Diabetes-Related Complications
3.6.1. Exercise Recommendation in Patients with Diabetic Nephropathy
Question 13: What exercise recommendations are needed in patients with diabetic nephropathy?
In patients at risk for diabetic nephropathy or with established kidney involvement, regular moderate to vigorous exercise reduces the incidence and progression of nephropathy, as well as the risk of cardiovascular events and mortality. Therefore, regular exercise should become a fundamental part of the management of individuals with diabetes, in the absence of contraindications (102). Waden et al. revealed in a prospective study of 1390 patients with diabetes that high-intensity physical activity may prevent the occurrence or advancement of nephropathy in individuals with type 1 diabetes (103). In conclusion, exercise should be recommended to patients with diabetic nephropathy, albeit with due precautions (26, 32, 102-104).
Recommendation 38: There is no need for any restrictions on the exercise of patients with diabetes with nephropathy. Since microalbuminuria and proteinuria are associated with an increased risk of cardiovascular diseases, exercise stress testing should be performed in sedentary patients before the start of exercise with an intensity higher than the activities of daily living (level D).
Recommendation 39: In dialysis patients, exercise is best done on days when the person is not on hemodialysis. However, exercise in patients with diabetes can be safely done even during hemodialysis sessions (using a sitting ergometer and preferably in the first half of the dialysis period to avoid hypotension) (level C).
Recommendation 40: Not only physical activity does not accelerate or progress nephropathy, but also especially vigorous-intensity exercise may exert positive preventive effects in individuals with type 1 diabetes (level B).
Recommendation 41: Electrolyte abnormalities (especially potassium and calcium), new changes in heart rhythm, weight gain of more than 4 kg compared to previous dialysis, pulmonary congestion, and peripheral edema are the contraindications for exercise in diabetic nephropathy (level C).
3.6.2. Exercise Recommendations in Patients with Diabetes-Related Cardiovascular Complication
Question 14: What exercise recommendations are needed in patients with cardiovascular complications?
A systematic review by Koivula et al. showed that exercise and lifestyle interventions can diminish cardiovascular risk factors and improve other health components in patients with type 2 diabetes. Therefore, it is reasonable to advise and encourage moderate-intensity physical activity in patients with diabetes, as long as it is designed according to the individual’s needs and health limitations and the individual is properly monitored by a clinician (105).
In another systematic review concerning individuals with type 1 diabetes, Wu et al. concluded that exercise plays a vital role in preventing cardiovascular diseases in type 1 diabetes. The analysis showed that exercise training may lead to positive effects on cardiovascular risk factors (including aerobic fitness), lipids, and insulin dosage. However, the ideal and minimal amounts of exercise to exert effective alteration in the cardiovascular risk profile of these patients remain to be elucidated (106).
In patients with coronary artery disease (CAD), higher-intensity aerobic or strength exercise may really enhance coronary perfusion. At least initially, a supervised exercise in a cardiac rehabilitation setting is suitable (32). Strength training of the lower-extremity increases the functional capacity in peripheral artery disease (PAD) (107). Low- or moderate-intensity walking and arm or leg ergometer are suitable aerobic modalities (32, 108). In general, exercise may have positive effects in the prevention and management of cardiovascular events in patients with diabetes, if prescribed and monitored properly (26, 32, 67, 100, 104-108).
Recommendation 42: Known cardiovascular disease is not an absolute contraindication to physical activity in patients with type 2 diabetes. Low-intensity physical activity is recommended for all peripheral vascular patients (level C).
Recommendation 43: Patients with cardiovascular autonomic neuropathy must be screened and evaluated before beginning any exercise program and require the physician clearance and exercise stress test to initiate an exercise program. The best way to determine the intensity of exercise in these patients is to use the RHR method with direct measurement of the maximum heart rate and with less accuracy the rate of perceived exertion (RPE) scale (level C).
Recommendation 44: Low to moderate-intensity exercise of the lower and upper extremity can be safely done in patients with diabetes with or without claudication with consideration of special cautions (level B).
3.6.3. Exercise Recommendation in Patients with Diabetic Retinopathy
Question 15: What exercise recommendations are needed in patients with diabetic retinopathy?
In a recent meta-analysis by Ren et al., authors revealed that physical activity has a slight protective association with retinopathy in patients with diabetes, and the impact was more pronounced on vision-threatening retinopathies (109). The amount of exercise or its components (time or intensity of each session) were not linked with severe retinopathy (110).
However, due to the risk of vitreous hemorrhage and retinal detachment in patients with unstable diabetic retinopathy, special exercise cautions are needed (32). In conclusion, the proper exercise program should be tailored for patients with diabetic retinopathy, especially in the mild and stable forms (26, 32, 73, 109, 110).
Recommendation 45: Moderate-intensity physical activity is associated with a lower risk of diabetic retinopathy and is likely to have a small protective effect (level A).
Recommendation 46: In patients with mild non-proliferative retinopathy, all activities are permitted, but yearly eye examination is necessary to check the progression. Patients with moderate non-proliferative disease should avoid activities, like powerlifting, which may significantly increase blood pressure. In cases of severe non-proliferative and unstable proliferative retinopathy, doing physical activities that may increase intraocular pressure and risk of hemorrhage (such as high intensity aerobic or strength exercise, head-down activities, breath-holding, jumping, and jarring) is forbidden. During a vitreous hemorrhage, no exercise should be done (level D).
3.6.4. Exercise Recommendations in Patients with Diabetic Neuropathy
Question 16: What exercise recommendations are needed in patients with diabetic neuropathy?
Long-term aerobic exercise may alter the natural history or even postpone the onset of peripheral neuropathy. Low-intensity aerobic training, could be efficient in the prevention and control of peripheral neuropathy. Exercise may have positive effects on both sensory and motor neuromuscular factors in individuals with diabetes (111).
Dixit et al. showed in a trial that moderate aerobic exercise might delay the progression of peripheral neuropathy in patients with type 2 diabetes (112). Kluding et al. also exhibited a significant decrease in pain and neuropathic symptoms and enhanced nerve fiber branching following a 10-week supervised aerobic and resistance training (113).
Regarding the effects of exercise on diabetic foot ulcer, Matos et al. showed in a systematic review that long-term exercise effectively lowers the risk of diabetic foot. Besides the standard medical care, exercise is recommended to decrease and manage this clinical state. Thus, a combined exercise program, including aerobic, strength, and balance exercises, may augment the benefits of standard care (114).
Autonomic neuropathy may influence multiple systems, particularly the cardiovascular system, which are essential for exercise adaptation. Precise evaluation of autonomic function, coronary artery disease, and exercise capacity is warranted. However, the need for pre-exercise evaluation should not impede exercise participation (115). Overall, exercise is not contraindicated in patients with diabetes with peripheral or autonomic neuropathy, but individualized assessment and special precautions should be considered (26, 32, 52, 111-118).
Recommendation 47: Patients with peripheral neuropathy and no acute foot ulcer can perform moderate-intensity weight-bearing exercises. Foot care, including appropriate footwear and regular foot inspection, is advised to prevent ulcers and enable early diagnosis. In patients with peripheral neuropathy, the risk of ulceration is not increased with moderate walking (level B).
Recommendation 48: In elderly patients with type 2 diabetes with decreased leg sensation or a history of foot ulcers and deformity, it is recommended to avoid high-intensity weight-bearing exercises (such as running and jumping movements) (level D).
Recommendation 49: Since there is no increase in leg ulcers in physically active patients compared to inactive individuals, exercise is not prohibited in patients with peripheral neuropathy (level B).
Recommendation 50: The existence of autonomic neuropathy may reduce the level of physical activity. Patients should observe special precautions to avoid troubles during activity (level C).
3.6.5. Exercise Recommendation in Other Diabetes-Related Complications
Question 17: What exercise recommendations are needed in patients with musculoskeletal or psychological complications?
Chronically elevated blood glucose levels may increase the risk of tendinopathy in patients with diabetes. Ranger et al. presented in a systematic review and meta-analysis that diabetes is associated with a higher risk of tendinopathy. In patients with diabetes, the progression of exercise load should be gradual to diminish the risk of tendinopathy. Furthermore, better control of diabetes may accelerate the rehabilitation process in individuals with diabetes (119).
The risk of depression in patients with diabetes is higher, compared to the non-diabetic individuals. Physical activity interventions showed a medium effect size in the management of diabetes-related depression. Therefore, physical activity interventions should be considered as a promising option to improve both depressive symptoms and biological outcomes (120). Generally, exercise should be considered for the prevention and management of other diabetes-related complications (32, 118-120).
Recommendation 51: Regular stretching exercises and a gradual increase in exercise intensity can reduce articular changes and diminish diabetes-related musculoskeletal complications (level C).
Recommendation 52: In elderly patients with type 2 diabetes who are depressed and functionally independent, regular exercise should be considered to improve mood and the quality of life (level A).
4. Conclusions
The current national guideline was developed to address important clinical issues regarding exercise prescription for Iranian patients with different types of diabetes. It emphasizes the importance of physical activity as an effective measure in the prevention and management of diabetes. The guideline provides evidence-based recommendations that may help physicians to prescribe exercise for Iranian patients with diabetes both safely and effectively.