1. Background
Nitrate is a competitive inhibitor of iodide (I) uptake at the thyroidal sodium-iodide symporter (NIS) (1). Nitrate, nitrite, and nitric oxide (NO) are the oxidized species of nitrogen available for biological uptake (2). Green leafy vegetables contain nitrate, and it is also added to foods as a preservative. Owing to the use of nitrate as an agricultural fertilizer and another atmospheric release of nitrogen oxides, environmental nitrogen emissions have increased enormously (3), leading to an increase in the nitrogen concentrations of surface waters as a potential water pollutant (1). NO is produced endogenously from L-arginine by NO synthases (NOS) enzyme family, mainly in vascular endothelial cells (4, 5). Thyroid hormones (THs) influence endothelium and directly rise NO production in vascular smooth muscle cells through the phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling pathway (5, 6). Direct measurement of this ubiquitous free radical signaling molecule is impossible due to its short half-life (< 0.1 s) (7). Hence, NO metabolites (nitrite + nitrate = NOx) are measured as indirect markers of serum NO synthesis in vivo (8, 9). Recent evidence shows the role of NO in the regulation of thyroid function, vascularity and blood flow (10, 11). Most of the studies focused on the disrupting effects of nitrate on the thyroid axis have considered diet and drinking water nitrate level rather than serum NOx concentration (12-14). In limited low sample size human studies, serum NOx concentration measurement in subjects with thyroid dysfunction reveals controversial results (5, 15-19). As far as we know, however, few studies indicate the correlation between serum NOx and changes in THs in an epidemiologic setting. Also, there is increasing evidence on the beneficial effects of high nitrate diets on hypertension control, diabetes mellitus management, etc. (20-23). Assessment of probable side effects of nitrate on human organs would be invaluable.
2. Objectives
Among the studies on nitrate as a thyroid disrupter, few have focused on serum nitrate level; therefore, the goal of the present study was to evaluate the correlation between serum NOx level and changes in free thyroxine (ΔFT4), thyroid-stimulating hormone (ΔTSH) and anti-thyroid peroxidase antibodies (ΔTPOAb) in a population-based cohort study.
3. Methods
3.1. Subjects
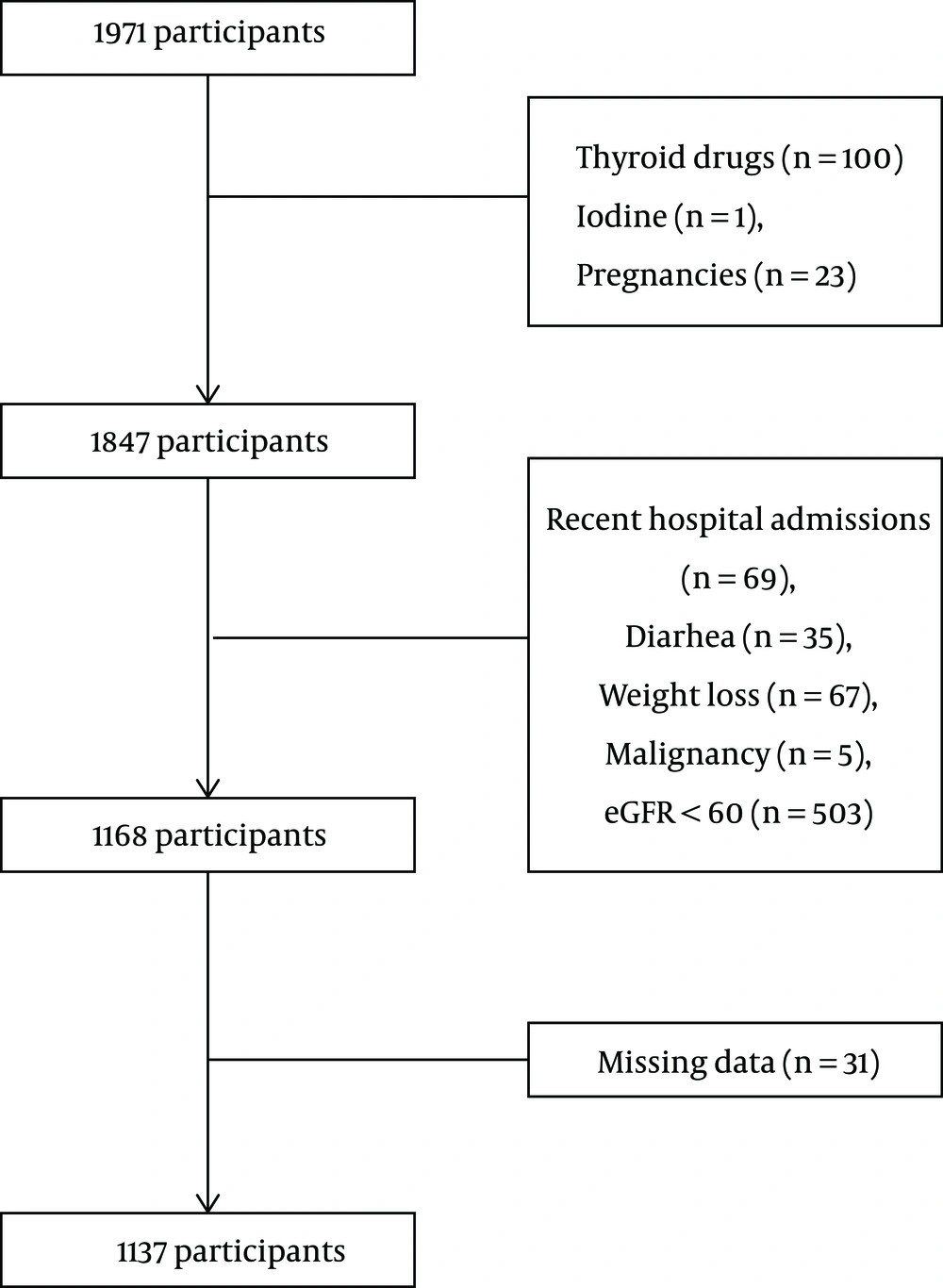
Tehran Thyroid study (TTS) is a cohort study being performed within the foundation of Tehran Lipid and Glucose study (TLGS). TLGS participants were a representative sample of Tehran (capital of Iran) population who were followed from 1999 during five phases and data on their smoking habits, radioiodine exposure, thyroid surgery, and thyroxin or anti-thyroid medications usage were collected (24, 25). This study included 1,971 participants over 20 years old from the third phase of TTS, whose data on serum NOx from the third phase and TSH, FT4, and TPOAb from the third and fourth phases were available. Members using thyroid and anti-thyroid drugs (n = 100), radioactive iodine (n = 1), pregnant women (n = 23), those with hospitalization history during the past three months (n = 69), chronic diarrhea (n = 35), weight loss (n = 67), malignancy (n = 5), estimated glomerular filtration rate < 60 ml/min/1.72 m2, and missing data (n = 13) in the third and fourth phases of TTS were excluded. Finally, 1,137 participants (515 men and 622 women) were included in the study (Figure 1). We calculated TSH, FT4, and TPOAb changes from the third to fourth phase and then evaluated the correlation between the third phase serum NOx level and thyroid function test changes.
Written informed consent was received from all contributors, and the Ethics Committee of the Research Institute for Endocrine Sciences approved this study.
3.2. Definition of Terms
Hyperthyroidism was defined as TSH < 0.324 mIU/L and FT4 > 19.95 pmol/L, hypothyroidism as TSH > 5.06 mIU/L and FT4 < 11.71 pmol/L, and subclinical hypothyroidism as TSH > 5.06 mIU/L and 11.71 pmol/L < FT4 < 19.95 pmol/L (26). TPOAb+ were men with TPOAb > 32 IU/ml and women with TPOAb > 35 IU/mL (27). History of cardiovascular disease (CVD) contained a history of heart surgery, myocardial infarction, angioplasty, a history of coronary care unit hospitalization, and cerebrovascular attack. Smoking was described as smoking one cigarette per day or more, or water-pipe use. Menopausal status was defined as no menstrual bleeding in the last 12 months.
3.3. Anthropometric, Clinical, and Laboratory Measurements
The method of data collection in the TTS has been mentioned in detail previously (25). Waist circumference (WC) and hip circumference (HC) were measured according to the standard TTS protocols. A standardized mercury sphygmomanometer was used for blood pressure (BP) measurement after a 15-minute relaxation in a sitting position. Blood sampling was performed after 12 h - 14 h overnight fasting. Samples were kept at -20°C until the biochemical measurements were done.
TPOAb was measured by immunoenzymometric assay (IEMA-Monobind, Costa Mesa, CA, USA) using the Sunrise ELISA reader (Tecan Co., Salzburg, Austria); inter and intra-assay CVs were 4.2% and 3.9%, respectively. Serum TSH and free T4 levels were quantified by the electrochemiluminescence immunoassay (ECLIA) method, using commercial kits on the Cobas e 411 analyzer (Roche Diagnostics GmbH, Mannheim, Germany). Inter- and intra-assay CVs were 4.6% and 1.4% for TSH, and 3.3% and 1.3% for free T4, respectively. Serum NOx level was quantified by the Griess method (28), which has been validated in our laboratory (29). In brief, serum was deproteinized using zinc sulfate (15 mg/mL) and centrifuged. Then 100 µL of the supernatant and 100 µL vanadium (III) chloride (Ardrich®) (8 mg/mL) were conveyed to a microplate well to reduce nitrate to nitrite (30). After Griess reagents addition and 30 minutes of incubation at 37°C, the absorbance was measured by the enzyme-linked immunosorbent assay (ELISA) reader (Tecan Co., Salzburg, Austria) at 540 nm. The linear standard curve established by 0 - 100 µM sodium nitrate was used to define serum NOX level. Inter- and intra-assay coefficients of variation (CV) were 5.2% and 4.4%, respectively. The enzymatic colorimetric method was used for fasting plasma glucose measurement by the glucose oxidase kit (Pars Azmoon Inc., Tehran, Iran); inter and intra-assay CVs were both 2.2%. Additionally, enzymatic colorimetric methods were used for triglyceride (TG) and total cholesterol (TC) measurement with glycerol phosphate oxidase and cholesterol esterase and cholesterol oxidase, respectively. TG and TC kits (Pars Azmoon Inc., Tehran, Iran) were used. Inter- and intra-assay CVs were 1.6% and 0.6% for TG and 2.0% and 0.5% for TC, respectively. Serum creatinine was quantified, using the photometric Jaffe method (Pars Azmoon Inc., Tehran, Iran). Inter- and intra-assay CVs were 3.1% and 2.2%, respectively. Moreover, eGFR was calculated using the following formula (31): eGFR = 186 × (serum Cr)-1.154 × (age)-0.203 × (0.742 if female).
3.4. Statistical Analysis
Qualitative data are presented as number and percentage. Normal distribution of the variables was evaluated using the Kolmogorov-Smirnov test. Quantitative data with non-normal distribution (creatinine, TG, and NOx) are showed as median and Interquartile range (IQR), while those with normal distribution are shown as mean ± standard deviation (SD). The differences in quantitative normal variables were evaluated by t-test, for non-normal variables using Mann-Whitney U-test, and for qualitative variables using chi-square test between men and women. Spearman’s correlation coefficients were computed to quantify the association between serum NOx and ΔTSH, ΔFT4, and ΔTPOAb. Association between serum NOx and ΔTSH, ΔFT4 and ΔTPOAb as well as between serum NOx tertiles and ΔTSH, ΔFT4, and ΔTPOAb in men and women was evaluated using linear regression analysis. The association between serum NOx and ΔTSH and ΔFT4 in TPOAb+ and TPOAb- men and women were also evaluated. We adjusted variables with a P-value for entry (PE) < 0.2 in the univariate analysis. The association between serum NOx level and the incidence of clinical and subclinical hypothyroidism in the fourth phase of TTS, which occurred in euthyroid participants of the third phase, was evaluated by logistic regression analysis. Two-tailed P-values < 0.05 were considered statistically significant. Owing to the differences in thyroid hormone levels and serum NOx in men and women, the data of these two groups were analyzed separately. The data were analyzed using SPSS (version 20.0) for windows.
4. Results
The characteristics of the study participants are represented in Table 1. Men, in comparison to women, were younger, had significantly higher levels of SBP, TG, FBS, Cr, WC, Waist/Hip Ratio (W/H Ratio), while lower values of HC. At baseline, 1,029 (90.7%) participants were euthyroid that TSH, FT4, and TPOAb were 1.7 (1.1 - 2.6) mIU/L, 15.3 ± 2.7 pmol/L, and 4.8 (2.9 - 9.3) IU/mL, respectively. Moreover, serum NOx was 30.71 ± 22.17 µmol/L.
| Parameters | Total (N = 1137) | Men (N = 515) | Women (N = 622) | P-Valueb |
|---|---|---|---|---|
| Age, y | 41.7 ± 12.7 | 43.0 ± 13.3 | 40.6 ± 12.1 | 0.001 |
| History of CVD, % | 34 (3.0) | 27 (5.2) | 7 (1.1) | < 0.001 |
| Menopausal status, % | 188 (30.2) | |||
| Smoking, % | 268 (23.6) | 237 (46.0) | 31 (5.0) | < 0.001 |
| Using medication, % | 296 (26.0) | 117 (22.7) | 179 (28.8) | 0.021 |
| Systolic blood pressure, mm Hg | 114.4 ± 17.4 | 119.1 ±16.0 | 110.6 ± 17.5 | < 0.001 |
| WC, cm | 91.1 ± 12.4 | 95.4 ± 10.3 | 87.5 ± 12.9 | < 0.001 |
| HC, cm | 101.2 ± 8.6 | 99 ± 6.9 | 102.9 ± 9.4 | < 0.001 |
| W/H ratio | 0.89 ± 0.09 | 0.96 ± 0.06 | 0.84 ± 0.08 | < 0.001 |
| BMI, kg/m2 | 27.3 ± 4.6 | 26.9 ± 4.3 | 27.6 ± 4.9 | 0.005 |
| Thyroid status | ||||
| Euthyroid, % | 1029 (90.7) | 484 (94.0) | 545 (87.6) | 0.421 |
| Hypothyroid, % | 20 (1.8) | 4 (0.8) | 16 (2.6) | 0.211 |
| Subclinical hypothyroid, % | 62 (5.5) | 17 (3.3) | 45 (7.2) | 0.252 |
| Hyperthyroid, % | 9 (0.8) | 3 (0.6) | 6 (1.0) | 0.534 |
| Subclinical hyperthyroid, % | 15 (1.3) | 7 (1.4) | 8 (1.3) | 0.865 |
| TSH, mIU/L | 1.7 (1.1 - 2.6) | 1.5 (1.0 - 2.2) | 1.9 (1.2 - 3.1) | < 0.001 |
| FT4, pmol/L | 15.3 ± 2.7 | 16.3 ± 2.9 | 14.7 ± 2.2 | 0.112 |
| TPOAb, IU/mL | 4.8 (2.9 - 9.3) | 4.4 (2.8 - 8.3) | 5.2 (3.0 - 11.8) | 0.029 |
| NOx, µmol/L | 24 (18 - 34) | 25 (19 - 34) | 24 (17 - 35) | 0.426 |
| Serum triglycerides, mg/dL | 130 (88 - 187) | 144 (103 - 204) | 116.5 (79 - 171) | < 0.001 |
| Total cholesterol, mg/dL | 188.7 ± 39.0 | 187.9 ± 37.2 | 189.4 ± 40.5 | 0.520 |
| Fasting serum glucose, mg/dL | 94.5 ± 26.3 | 96.4 ± 28.0 | 93.0 ± 24.6 | 0.029 |
| Serum creatinine, mg/dL | 1.0 (0.9 - 1.1) | 1.14 (1.1 - 1.2) | 0.9 (0.9 - 1.0) | < 0.001 |
Abbreviations: BMI, body mass index; CVD, cardiovascular disease; FT4, free thyroxin; HC, hip circumference; NOx, serum nitrite + nitrate; TPOAb, thyroid peroxidase antibody; TSH, thyroid-stimulating hormone; WC, waist circumference; W/H R, waist/hip ratio.
aValues are expressed as mean ± SD or median (IQR) and categorical variables as percent.
bFor continuous variables independent t-test and for categorical one chi-square test were used.
No correlations were found between serum NOx and ΔTSH (P = 0.350 in men and P = 0.142 in women); serum NOx and ΔFT4 (P = 0.723 in men and P = 0.557 in women). Also, there was no correlation between serum NOx and ΔTPOAb (P = 0.188 in men and P = 0.734 in women) after multivariable adjustment after three years of follow-up (Table 2). This study showed no association between serum NOx tertiles and ΔTSH, ΔFT4 and ΔTPOAb in men and women before and after multivariable adjustment (data not shown). We repeated our analysis in TPOAb+ and TPOAb- participants separately and found no association before and after multivariable adjustment (Table 2).
| Parameters | Men | Women | ||||
|---|---|---|---|---|---|---|
| B (SE) | P-Value | R-Square | B (SE) | P-Value | R-Square | |
| ΔTSH | ||||||
| Unadjusted | ||||||
| Total | -0.907 (0.970) | 0.350 | 0.002 | 4.236 (2.884) | 0.142 | 0.003 |
| TPOAb+ | -0.275 (1.030) | 0.791 | 0.041 | 0.042 (1.156) | 0.971 | 0.004 |
| TPOAb- | -0.947 (1.047) | 0.366 | 0.042 | 4.612 (3.290) | 0.162 | 0.061 |
| Multivariable adjusteda | ||||||
| Total | -0.776 (0.966) | 0.423 | 0.017 | 4.948 (2.919) | 0.091 | 0.009 |
| TPOAb+ | 0.250 (0.998) | 0.603 | 0.143 | -0.262 (1.154) | 0.821 | 0.093 |
| TPOAb- | -0.828 (1.043) | 0.427 | 0.016 | 5.402 (3.275) | 0.100 | 0.046 |
| ΔFT4 | ||||||
| Unadjusted | ||||||
| Total | 0.025 (0.072) | 0.723 | < 0.001 | -0.014 (0.024) | 0.557 | 0.001 |
| TPOAb+ | 0.013 (0.945) | 0.089 | < 0.001 | 0.001 (0.072) | 0.984 | < 0.001 |
| TPOAb- | 0.038 (0.019) | 0.040 | 0.095 | -0.018 (0.025) | 0.483 | 0.031 |
| Multivariable adjusteda | ||||||
| Total | 0.020 (0.071) | 0.778 | 0.019 | -0.024 (0.024) | 0.312 | 0.012 |
| TPOAb+ | -0.244 (1.013) | 0.611 | 0.154 | -0.011 (0.071) | 0.873 | 0.067 |
| TPOAb- | 0.036 (0.018) | 0.053 | 0.024 | -0.024 (0.025) | 0.341 | 0.015 |
| ΔTPOAb | ||||||
| Unadjusted | ||||||
| Total | -0.208 (0.158) | 0.188 | 0.003 | -0.093 (0.273) | 0.734 | < 0.001 |
| TPOAb+ | 0.107 (0.994) | 0.915 | < 0.001 | -0.298 (0.402) | 0.460 | 0.006 |
| TPOAb- | -0.208 (0.150) | 0.166 | 0.004 | -0.097 (0.306) | 0.762 | < 0.001 |
| Multivariable adjusteda | ||||||
| Total | -0.202 (0.159) | 0.205 | 0.006 | 0.013 (0.274) | 0.961 | 0.015 |
| TPOAb+ | 0.296 (0.991) | 0.767 | 0.066 | -0.276 (0.394) | 0.485 | 0.052 |
| TPOAb- | -0.208 (0.150) | 0.166 | 0.004 | 0.023 (0.307) | 0.941 | 0.020 |
aAdjustment was done for age, systolic blood pressure, smoking status, menopausal status, waist circumference, using medications, serum triglyceride, serum total cholesterol, fasting serum glucose, and serum creatinine. TPOAb+: > 32 IU/mL for men and > 35 IU/mL for women.
Out of all euthyroid participants in the third phase, 10 subjects (0.94%) were diagnosed as clinical hypothyroid and 43 (4.04%) as subclinical hypothyroid in the fourth phase. There was no association between serum NOx tertiles and the incidence of clinical and subclinical hypothyroidism in participants after three years of follow-up (Table 3).
| Parameters | Clinical Hypothyroidism | Subclinical Hypothyroidism | Clinical + Subclinical Hypothyroidism | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P-Value | OR (95% CI) | P-Value | OR (95% CI) | P-Value | |
| Unadjusted | ||||||
| Tertile 1 (ref) | ||||||
| Tertile 2 | 1.327 (0.353 - 4.983) | 0.675 | 1.284 (0.637 - 2.590) | 0.485 | 1.300 (0.695 - 2.433) | 0.411 |
| Tertile 3 | 0.259 (0.029 - 2.325) | 0.227 | 0.686 (0.304 - 1.549) | 0.365 | 0.591 (0.277 - 1.260) | 0.173 |
| Adjusteda | ||||||
| Tertile 1 (ref) | ||||||
| Tertile 2 | 1.468 (0.388 - 5.551) | 0.572 | 1.687 (0.813 - 3.498) | 0.160 | 1.658 (0.866 - 3.172) | 0.127 |
| Tertile 3 | 0.310 (0.034 - 2.811) | 0.298 | 0.886 (0.383 - 2.052) | 0.778 | 0.747 (0.343 - 1.624) | 0.461 |
Abbreviation: OR, odds ratio.
aAdjustment was done for age, systolic blood pressure, serum triglyceride, serum creatinine, waist circumference, smoking status.
5. Discussion
This study showed no association between serum NOx concentration, and ΔTSH, ΔFT4, and ΔTPOAb in a three-year follow-up. Serum NOx level was within the normal range for the general population in our participants. Neither was any association demonstrated between serum NOx concentration and incidence of clinical and subclinical hypothyroidism in euthyroid participants at the end of the follow-up.
These findings are inconsistent with those of the Bagherpoor et al. (32), in a cross-sectional study conducted on 1,771 participants in the third phase of TTS for evaluating the association between serum NOx concentration and thyroid function tests. This study showed a significant correlation between serum NOx and FT4 in men, and TPOAb in women. These conflicting results may be due to several factors such as differences in the study design and participants. Additionally, unlike the study conducted by Bagherpoor et al. (32), the participants with eGFR < 60 ml/min/1.72 m2 were excluded from this study since individuals with lower eGFR have higher serum nitrate levels (33) and various thyroid function test abnormalities (34). Some other studies, in which serum NO level was evaluated in hypo- and hyperthyroid individuals, have shown controversial results. Verma et al. (35) showed higher serum NO levels in 50 hypothyroid individuals in India, which was similar to Atta et al. (18) and Coria et al. studies (17) conducted on 60 and 20 hypothyroid cases, respectively. In contrast, other studies showed lower serum NO level (5) or no nitrate changes in hypothyroid patients (15). Results on NO level in hyperthyroid patients are controversial (15, 35). All of these studies had small sample sizes and were carried out in different countries with different statuses of iodine sufficiency.
Despite other studies discussed herein, this is a cohort study with a larger sample size designed differently. Some studies were done in iodine-deficient areas, whereas Iran has been an iodine-sufficient area since the year 2000.
In vivo nitrate at a molar concentration of 297 µmol/L corresponds to 50% inhibition of iodide (I) uptake through human NIS expressing in Chinese hamster ovary cells in a competitive manner (36). The 95% reference value for serum NOx concentration is 11.5 - 76.4 µmol/L in men and 10.1 - 65.6 µmol/L in women, which corresponds to 10-15% inhibition of I uptake with NIS in vivo (30). With respect to Km = 33.9 µmol/L for I uptake at NIS level and very low serum I concentrations in human (2 - 10 µg/L in iodine-sufficient areas like Iran), theoretically, nitrate can block I uptake at the thyroidal sodium-iodide symporter (NIS) in normal nitrate levels in human (36), which is more probable in iodine-deficient areas (1).
According to our results, it seems that serum nitrate at normal levels in humans is not potent enough to reduce TH production in iodine-sufficient areas. Several studies have demonstrated the beneficial effects of high-level nitrate diets such as dietary approaches to stop hypertension (DASH) on the reduction of endothelial dysfunction, cardiovascular treatment (22), hypertension control (21), declining insulin resistance (20), and the improvement of brain perfusion in older adults (23). Moreover, nitrate-containing drugs are widely used for the treatment of cardiovascular diseases. Our results suggest that these treatments have no adverse effects on thyroid function tests if they maintain serum NOx levels within normal range.
Regarding study strengths, to the best of our knowledge, this is one of the few population-based cohort studies evaluating the correlation between serum NOx concentration and ΔTSH, ΔFT4 and ΔTPOAb in more than 1,000 participants over a three-year follow-up. However, we had some limitations: First, we did not assess the nitrate content of participant’s diet as an important source for nitrate in humans (37); however, the subjects blood samples were obtained after 12 - 24 hours of overnight fasting and awareness that dietary nitrate had to be removed from the plasma within 12 hours of a meal (38). In addition, the majority of nitrate is produced via enzymatic NO formation from NOS during fasting (39). Second, the history of immune disease, which can affect serum NOx concentration, was not registered (40). Third, since we only had data on serum NO level in the third phase, its changes were not calculated; however, the factors affecting serum NO level such as smoking, BMI, FBS, eGFR, etc. were considered (41). Finally, it seems that three years of follow-up may not be sufficient for the assessment of hypothyroidism incidence.
5.1. Conclusions
This study showed no association between serum NOx level and thyroid function tests (TSH, FT4, and TPOAb) changes and no correlation between serum nitrate levels and the incidence of clinical and subclinical hypothyroidism during three years of follow-up. We recommend planning a larger sample size cohort study with longer follow-up, which can better assess the relationship between serum NOx level and the incidence of hypothyroidism and hyperthyroidism. It may also be reasonable to assess this correlation in populations with high serum nitrate levels from iodine-deficient areas.