1. Background
The 5 alpha-reductase type 2 enzyme converts testosterone to the more potent androgen dihydrotestosterone (DHT). In male fetuses, DHT is responsible for external genitalia differentiation, including midline fusion, urogenital tubercle, and fold elongation and enlargement, and also plays a role in prostate development.
The SRD5A2 gene, which encodes the DHT enzyme, is located at chromosome 2p23.1. Various defects at the SRD5A2 gene have been reported to manifest clinically as 5 alpha-reductase type 2 deficiency. This condition is an autosomal recessive disorder characterized by variable degrees of under virilization in affected 46,XY individuals, ranging from micropenis, hypospadias, and genitalia ambiguity to the female external genitalia. Individuals with the classic phenotype are usually reared as female and become virilized during puberty. This may lead to gender changes and sociopsychological problems (1-3). Approximately 55% - 63% of 5ARD2 patients chose to be reassigned as male (4).
Since the reporting of the initial cases (3, 5, 6), there have been many reported 5ARD2 cases around the world (7-15), including Indonesia (16-18). For the diagnosis of 5ARD2, the testosterone/dihydrotestosterone (T/DHT) ratio is primarily used by clinicians in our country before ordering the molecular confirmation test. However, the DHT test is not readily available and has to be sent abroad, resulting in increased costs and delays in 5ARD2 diagnosis. Furthermore, the T/DHT ratio was reported to be inconclusive in detecting 5ARD2 cases, and currently, the consensus on the cutoff to interpret the ratio is lacking (14, 19).
Urinary steroid profiling (USP) is known to have a significant role in diagnosing several disorders affecting steroid metabolism, including congenital adrenal hyperplasia, Cushing syndrome, 17 beta-hydroxysteroid dehydrogenase, 3 beta-hydroxysteroid dehydrogenase, and 5 alpha-reductase type 2 deficiency (5, 6, 20, 21). Chan et al. (19) reported convincing results of USP using ratios of urinary 5β/5 steroid metabolites, including etiocholanolone/androsterone (Et/An), in detecting 15 cases of 5ARD2 and thereby, proposed the use of USP in the diagnostic algorithm of 5ARD2, replacing the use of the T/DHT ratio and molecular analysis. Furthermore, according to reports by Imperato-McGinley et al. (22), the urinary 5β/5α steroid metabolite ratios, including the Et/An ratio, might be useful in discriminating carriers of 5ARD2 and normal individuals. Since the Et/An ratio has never been employed in the diagnosis of 5ARD2 cases and carriers in our country, it is warranted to have supported data from our series.
2. Objectives
We aimed to analyze the diagnostic value of the urinary Et/An ratio versus T/DHT ratio in detecting 5ARD2 patients and carriers.
3. Methods
The study was conducted concurrently with another study to characterize molecular defects in our 5ARD2 cases. The main results of the molecular study were published elsewhere (18) and revealed 37 of our 46,XY DSD subjects had pathological variations of the SRD5A2 gene in both of their alleles. Among those subjects, 20 carried severe mutations, while 17 had benign mutations (the p.Val89Leu).
3.1. Study Population
We recruited 46,XY DSD individuals, who were suspected of having 5ARD2, and their eligible close family members (parents, siblings, aunts, uncles, and grandparents) as study participants. We excluded subjects, who underwent external and/or internal genitalia surgery without a written record on their clinical phenotypes, who had steroid treatment within four weeks before hormonal tests, and who had renal dysfunctions. Subjects’ characteristics on age, age at diagnosis, sex rearing, gender change, and pubertal stages were collected. The evaluation of external genitalia appearances (the phallus size, the location of gonads and urethral meatus, and the presence/absence of labioscrotal fusion) was performed using the external genitalia masculinization score (EMS) as defined earlier by Ahmed et al. (23).
The DNAs of the study participants were analyzed for SRD5A2 gene defects. Hormonal profiles, including T, DHT, and the urinary Et/An ratio, were also obtained. Furthermore, to compare the diagnostic value of the T/DHT and urinary Et/An ratios in diagnosing 5ARD2 patients and carriers, we used receiver operating characteristic (ROC) curve analysis by employing molecular analysis of the SRD5A2 gene as the gold standard. The study protocol followed the ethical standards of the Health Research Ethics Committee of the Faculty of Medicine, Universitas Indonesia, with regards to the protection of human rights and welfare in medical research with approval number 576/UN2.F1/ETIK/2016.
3.2. SRD5A2 Gene Analysis
The extraction of genomic DNA from peripheral blood leucocytes was performed using standard procedures (24). Amplification of exon 1 - 5 and flanking regions of the SRD5A2 gene was carried out using primer sequences derived from Nie et al. (25) with modification at exon 4 forward primer, which was sequenced as follows: 5’-CCA AGA GGA TTC CAC CAA ACT C-3’. Further SRD5A2 gene analysis was conducted following our previous report (18). The published sequence (GenBank: NG-008365.1) was used as a reference.
3.3. Hormonal Assays
The human chorionic gonadotrophin (hCG) stimulation test was performed as previously described (18) in five prepubertal patients with unpalpable testes. We performed the competitive ELISA methods as described in our previous report (18) using the TOSOH AIA-900 automated immunoassay analyzer to examine the serum testosterone (ST AIA-PACK testosterone) and commercial kit of DH-optimized ELISA for DHT test (DRG Instruments GmbH, Germany, ref. EIA-5761). The urinary Et and An analyses were carried out using gas chromatography-mass spectrometry (GC-MS) with an Agilent 7890 gas chromatographer (Agilent technologies) coupled with a mass spectrometer (model 5975, Agilent technologies). Details of the procedures were described earlier in our simultaneous study (18).
4. Results
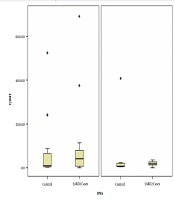
Sixty-six suspected 5ARD2 patients (age one month to 42 years) and 95 family members (age 36.3 ± 12.5 years) gave consent and took part in the study. Among the patient group, 37 were confirmed to have SRD5A2 gene variants in both alleles, and 53 family members were detected as carriers. A total of 46,XY DSD patients who were normal or heterozygous for SRD5A2 gene mutations (29 subjects) were included as controls in the ROC curve analysis for the patient group, and 42 family members who were normal for the SRD5A2 gene were recruited as controls in the ROC analysis for the carrier group. The characteristics of the subjects are summarized in Tables 1 and 2. Among the patient group, age, age at diagnosis, sex rearing, and pubertal stage were not significantly different between the patient and control groups, nor was the T/DHT ratio (Table 1). The EMSs were also comparable between the two groups. However, the urinary Et/An ratio was significantly different between the two subgroups in both patients (Table 1) and carrier groups (Table 2).
| Characteristics | 46,XY DSD Patients (N = 66) | P | |
|---|---|---|---|
| 5ARD2 Patients (N = 37) | Controls (N = 29) | ||
| Age, y | 8.3 (0.1 - 41.8) | 5.3 (0.1 - 9023.9) | 0.105 |
| ≤ 0.4 | 2 | 2 | |
| > 0.4 - < 9 | 18 | 17 | |
| ≥ 9 - < 18 | 6 | 8 | |
| ≥ 18 | 11 | 3 | |
| Age at diagnosis, y | 6 (0 - 41.78) | 4.5 (0 - 23.9) | 0.713 |
| Sex rearing | 0.815 | ||
| Male | 24 | 19 | |
| Female | 13 | 11 | |
| Pubertal stage | 0.273 | ||
| Prepubertal | 22 | 22 | |
| Pubertal | 15 | 8 | |
| Gender change | 10 | 4 | 0.05 |
| EMS | 3 (0 - 11) | 3 (0 - 10.5) | 0.228 |
| T/DHT ratio | 23.64 (0.1 - 70000) | 9.5 (2.18 - 38000) | 0.286 |
| Et/An ratio | 1.19 (0.03 - 12.23) | 0.52 (0.06 - 2.07) | < 0.001 |
Abbreviations: 5ARD2, 5 alpha-reductase deficiency; An, androsterone; DHT, dihydrotestosterone; EMS, external genitalia masculinization score; Et, etiocholanolone; T, testosterone.
| Characteristics | Family Members (N = 95) | P | |
|---|---|---|---|
| 5ARD2 carriers (N = 53) | Controls (N = 42) | ||
| Age, y | 37.5 ± 14.5 | 37.5 ± 10.5 | 0.137 |
| ≤ 0.4 | 0 | 0 | |
| > 0.4 - < 9 | 2 | 1 | |
| ≥ 9 - < 18 | 5 | 0 | |
| ≥ 18 | 46 | 41 | |
| Sex | 0.353 | ||
| Male | 19 | 18 | |
| Female | 34 | 24 | |
| Pubertal Stage | 0.160 | ||
| Prepubertal | 5 | 1 | |
| Pubertal | 48 | 41 | |
| Children | 2 (0-6) | 2 (0-3) | 0.317 |
| T/DHT ratio | 6.77 (0.3-500) | 5.05 (0.31-31.11) | 0.379 |
| Et/An ratio | 1.28 (0.08-17.56) | 0.76 (0.1-5.4) | < 0.001 |
Abbreviations: 5ARD2, 5 alpha-reductase type 2 deficiency; An, androsterone; DHT, dihydrotestosterone; Et, etiocholanolone; T, testosterone.
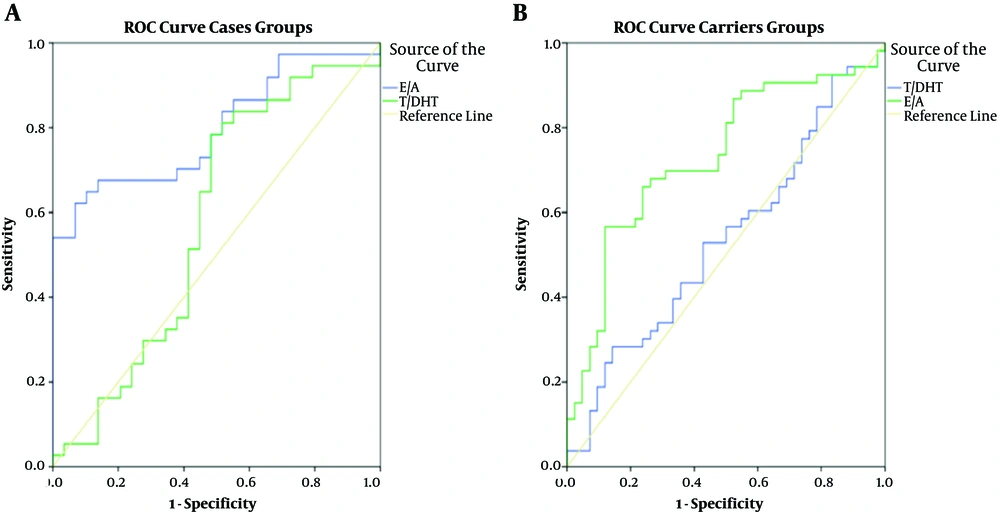
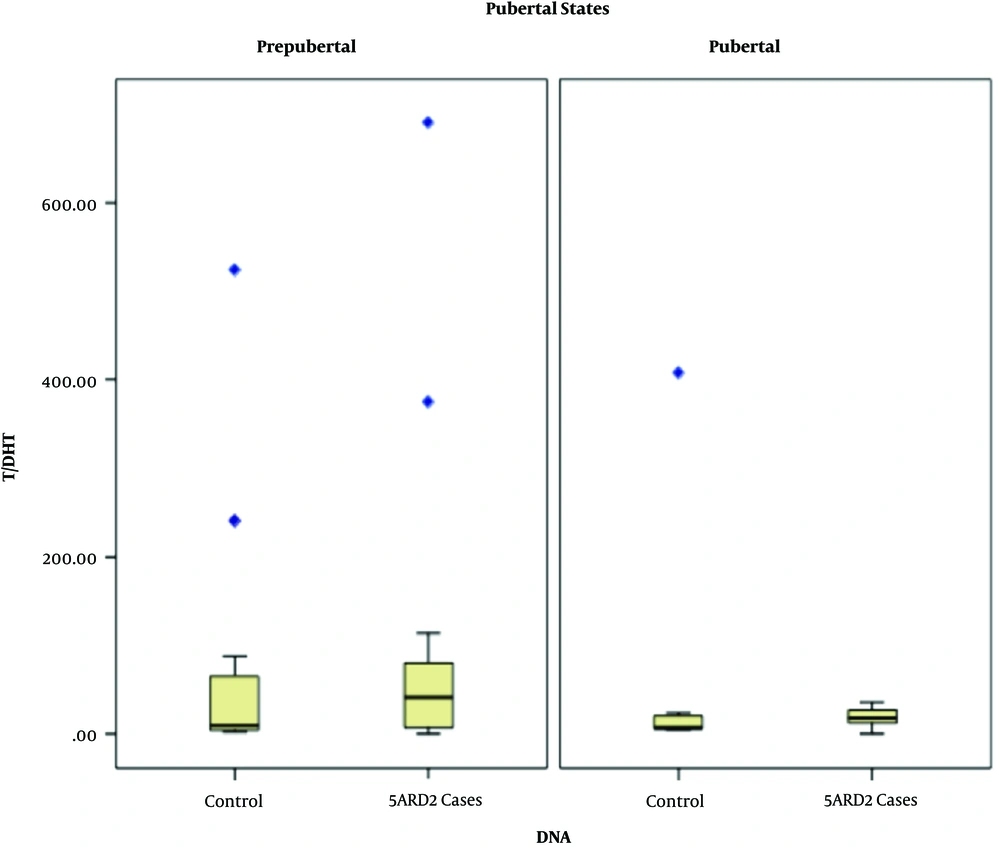
Comparisons of the ROC curves of the T/DHT and urinary Et/An ratios for both case and carrier groups are shown in Figure 1A and B, respectively. Surprisingly, the AUC (area under the curve) for the T/DHT ratio was inconclusive for 5ARD2 diagnosis in both patient and carrier groups. The AUC of the T/DHT ratio was 57.7% (95% CI 43.0 - 72.4%, P > 0.05) in diagnosing 5ARD2 patients and 54.1% (95% CI 42.4 - 65.8%, P > 0.05) in diagnosing carriers. This result was similarly demonstrated in both prepubertal and postpubertal patients (Figure 2). On the other hand, the urinary Et/An ratio had an AUC of 79.7% (95% CI 69.0 - 90.4%, P < 0.001) in discriminating the patient group and 75.1% (95% CI 65.1 - 85.1%, P < 0.001) in the carrier group. Subsequently, the cutoff values of the urinary Et/An ratio in diagnosing SRD5A2 patients and carriers were determined using linear graphs based on the ROC curve data and were found to be ≥ 0.95 in detecting 5ARD2 patients and ≥ 0.99 in detecting carriers. The diagnostic values of the urinary Et/An ratio using cutoffs found in this study are compared with the reference values used by Chan et al. (19) in Table 3.
| Sensitivity, % | Specificity, % | PPV | NPV | Accuracy, % | |
|---|---|---|---|---|---|
| Patient group | |||||
| Cutoff ≥ 0.95 | 67.57 | 86.2 | 82.61 | 67.57 | 75.76 |
| Reference values* | 48.65 | 6.90 | 40.0 | 9.52 | 30.30 |
| Carrier group | |||||
| Cutoff ≥ 0.99 | 67.92 | 73.81 | 76.60 | 64.58 | 70.53 |
| Reference valuesa | 35.85 | 88.10 | 79.17 | 52.11 | 58.95 |
Abbreviations: NPV, negative predictive value; PPV, positive predictive value.
aReported by Chan et al. (19).
5. Discussion
An increase in the T/DHT ratio is a biochemical marker broadly used in clinical settings to diagnose 5ARD2. There have been many studies elaborating the role of the T/DHT ratio in diagnosing 5ARD2; however, some have reported conflicting results (14, 19, 26) due to false-negative responses or variations in determining cutoff values. Bertelloni et al. (27) reported that the T/DHT ratio was more profound after the hCG test. Variable cutoff values of the T/DHT ratio have yielded a wide range of sensitivity and specificity in 5ARD2 diagnosis. Despite these issues, the T/DHT ratio is mainly employed in clinical practice.
The accuracy of the T/DHT ratio obtained in this study was beyond our expectations (Tables 1 and 2, and Figure 1). First, we considered that not performing the hCG stimulation test for all prepubertal patients was the cause of this result. However, after analyzing the ratio separately by pubertal stage, the result was similar (Figure 2). In contrast, the urinary Et/An ratio revealed promising results. The urinary Et/An ratio is the only test available for assessing the urinary 5β/5α steroid metabolite ratio in Indonesia. Despite the low sensitivity of this ratio in detecting 5ARD2 patients and carriers, having this result may add evidence to implement the ratio in clinical practice, especially if molecular diagnostic services are not accessible.
Since we did not have any reference values for our normal individuals, we applied cutoff values, which were determined by a linear graph of sensitivity and specificity from the ROC curve data. Using these cutoff values for detecting 5ARD2 patients (≥ 0.95) and carriers (≥ 0.99), the sensitivity and specificity of the urinary Et/An ratio for diagnosing patients were 67.57% and 86.2%, respectively, while for diagnosing carriers, the sensitivity was 67.92% and the specificity was 73.81%. These results were lower than those in other published studies (19, 21, 28). This discrepancy may have been caused by different study methods, the severity of the cases included, and lack of controls in the studies. Twelve of the 16 5ARD2 patients reported by Chan et al. (19) carried deteriorating mutations in both alleles. Meanwhile, in Lucas-Herald et al.’s report (21), only two 5ARD2 patients, who lacked data on molecular defects, were detected among 84 patients. Approximately half of the cases in our series were mild (17 out of 37), which may have led to equivocal hormonal results (18). This might also explain the insignificant difference in cutoff values of the ratios for detecting patients and carriers. The p.Val89Leu variation in the SRD5A2 gene is considered a polymorphism because it is also detected in normal individuals. However, several studies have reported its specific characteristics, which could decrease enzymatic activity by 30% (9, 29, 30), and further reduction may occur if it is combined with other damaging SRD5A2 gene mutations.
In diagnosing 5ARD2, we proposed to use cutoff values of the urinary Et/An ratio based on the diagnostic value calculated in Table 3 instead of the reference values. Considering the influence of ethnic background on urinary metabolite ratios (31), we used the reference value determined by Chan et al. (19) to make a comparison with our results, as our patients were Asian. Using the cutoffs determined in this study, the sensitivity and accuracy of the test yielded better results.
The urinary Et/An ratio showed interesting results. Although the diagnostic value did not appear sufficiently convincing, we believe this may lead to a more obvious path in diagnosing carriers, especially when other 5β/5α steroid metabolite ratios are added. This study added informative data with cutoffs of the metabolite ratio, which may be implemented in clinical settings. To date, there have been no tests that can determine 5ARD2 carriers, except USP and molecular analysis. Since molecular analysis is not available in most regions in our country and still considered costly, the urinary Et/An ratio is an alternative test in detecting more patients and carriers and may contribute to genetic counseling of 5ARD2 patients.
5.1. Conclusions
The testosterone/DHT ratio was inaccurate in diagnosing 5ARD2 patients and carriers. When molecular analysis for the SRD5A2 gene is lacking, the urinary Et/An ratio may be a useful test to diagnose 5ARD2 patients and carriers.