1. Background
Hashimoto’s thyroiditis (HT) is one of the most common causes of hypothyroidism and placed in the category of autoimmune thyroid disease (AIT), which is one of the most prevalent autoimmune diseases and is considered a risk factor for papillary carcinoma and thyroid cancer (1-3). Currently, Levothyroxin (LT4) is prescribed for the treatment of HT. This drug is just prescribed to compensate for the effects of hormone reduction; however, it is not able to prevent the destructive effects of autoreactive immune cells. At present, there is no specific treatment modality to suppress autoimmune destruction. Therefore, LT4 is usually treated for controlling HT, not for definitive treatment (4, 5).
Understanding other specific immune mechanisms involved in the pathogenesis of HT could help us find some targeted therapies such as monoclonal antibodies against surface antigens, cytokines, cytokine receptors, and intracellular signaling molecules, which would improve the treatment efficacy (6). CD4+ T cells play an important role in the regulation of immune responses and protecting a balance between pro- and anti- inflammatory responses (7). Increasing Th17 cells is one of the pathogenetic mechanisms in HT. Due to the role of these cells in induction of inflammatory conditions, the pathogenetic role of these cells has been determined (7, 8).
On the other hand, the decrease in the cell population of regulatory T lymphocytes is known as another major factor in the pathogenesis of HT. This cell population prevents the development of autoimmune diseases by reducing the activity and number of autoreactive cells (9). In HT disease, T cells shift toward Th1 cells, and CD8+ T lymphocytes have also been observed, which can produce IFN-γ and express Fas-L on their surface, which result in the destruction of thyroid cells. On the other hand, autoantibodies can damage thyroid cells via antibody-dependent cell-mediated cytotoxicity (ADCC) and complement fixation (8, 10, 11). The production of antibodies against TG can be induced by extensive destruction of the thyroid gland (12). Therefore, we used the high TG titer criterion to classify patients in terms of disease severity.
In the present study, CD4+ T cells of people with HT were evaluated in two separate groups and compared with normal people. The serum level of anti-TPO was above 100 IU/mL in the group 1 (n = 13), whereas serum levels of both anti-TPO and anti-TG were above 100 IU/mL and the group 2 (n = 13), and normal women (group 3) had anti-TG and anti-TPO under 100 IU/mL.
2. Methods
2.1. Patients and Study Design
Thirty-six women with HT, aged 20 - 45 years, participated in this study (Table 1). Hashimoto’s thyroiditis disease was diagnosed based on the presence of anti-TG and/or anti-TPO antibodies in patients’ sera, abnormal thyroid function, enlarged thyroid gland, and morphological changes in thyroid ultrasound (13, 14). The patients were referred to this study by the endocrinologists of Erfan Endocrine Clinic and Emam Khomeini Hospital from November 1, 2016, to April 1, 2017. None of the patients were treated with anti-inflammatory drugs before and during the study. Patients with a history of other special diseases, including autoimmunity, malignancy, diabetes, chronic kidney or liver disease, were not enrolled in the study.
| Variables | Anti-TPO > 100 and Anti-TG > 100 (n = 17) | Anti-TPO > 100 (n = 16) | Healthy Women (n = 13) |
|---|---|---|---|
| TSH (μIU/mL) | 5.1 (7.8) | 3.1 (2.4) | 2.5 (0.9) |
| Anti-TPO (IU/mL) | 354.9 (128) | 254.1 (84.8) | 16 (7.3) |
| Anti-TG (IU/mL) | 1004.5 (1247.6) | 48.5 (23.3) | 39.3 (14) |
| Dosage of levothyroxin (μg/kg/day) | 1 (0.0) | 1.07 (0.0) | 0 |
The patients were divided into two groups. Group 1 (n = 13) consisted of patients with the serum level of anti-TPO more than 100 IU/mL, whereas group 2 (n = 13) consisted of patients who had both anti-TPO and anti-TG more than 100 IU/mL in their sera. The healthy women (n = 11) were considered the control group (group 3).
2.2. Peripheral Blood Mononuclear Cells (PBMCs) and CD4+ T Cells Isolation
Fifteen mL of fresh blood samples were collected from all participants into tubes containing EDTA. Subsequently, PBMCs were separated by standard Ficoll-Hypaque (Pharmacia-Amersham, Piscataway, density 1.077 g/mL) density-gradient centrifugation. CD4+ T cells were purified from fresh PBMCs using magnetic-activated cell sorting (MACS) (human CD4+ T Cell Isolation Kit, Miltenyi) via depletion of non-target cells, a negative selection. Briefly, 4 × 105 CD4+ T cells were seeded into 24-well plates in 1 mL of complete culture medium (RPMI 1640 supplemented with 2mM glutamine, 10% heat-inactivated fetal calf serum, 100 U/mL penicillin, 100 μg/mL streptomycin) and incubated at 37°C in 5% CO2 for 16 - 18 hours. Then cells were stimulated for 6 hours with cell stimulation cocktail plus protein inhibitor that were purchased from eBioscience (San Diego, CA, USA). This cocktail contains phorbol myristate acetate (PMA 50ng/mL), ionomycin (1 μg/mL) monensin (1.4 μg /mL) and brefeldin A (3 μg/mL). After 6 hours incubation, the cells were transferred to 5-mL sterile tubes and washed once in phosphate-buffered saline (PBS) and centrifuged at 400 × g for 5 min.
2.3. Flow Cytometry Analysis
All antibodies and buffers were purchased from eBioscience (San Diego, CA, USA). For intracellular staining, the cells were washed, fixed, permeabilized, and incubated with monoclonal antibodies against IL-17 (PerCP-Cyanine5.5, eBio64dec17, IgG1, κ; eBioscience, San Diego, CA), IFN-γ (FITC, 4S.B3, IgG1, κ; eBioscience), IL-4 (APC, 8D4-8, IgG1, κ; eBioscience), and IL-10 (PE, JES3-9D7, IgG1, κ; eBioscience). Fixation and permeabilization by buffers were performed according to the manufacturer’s instructions.
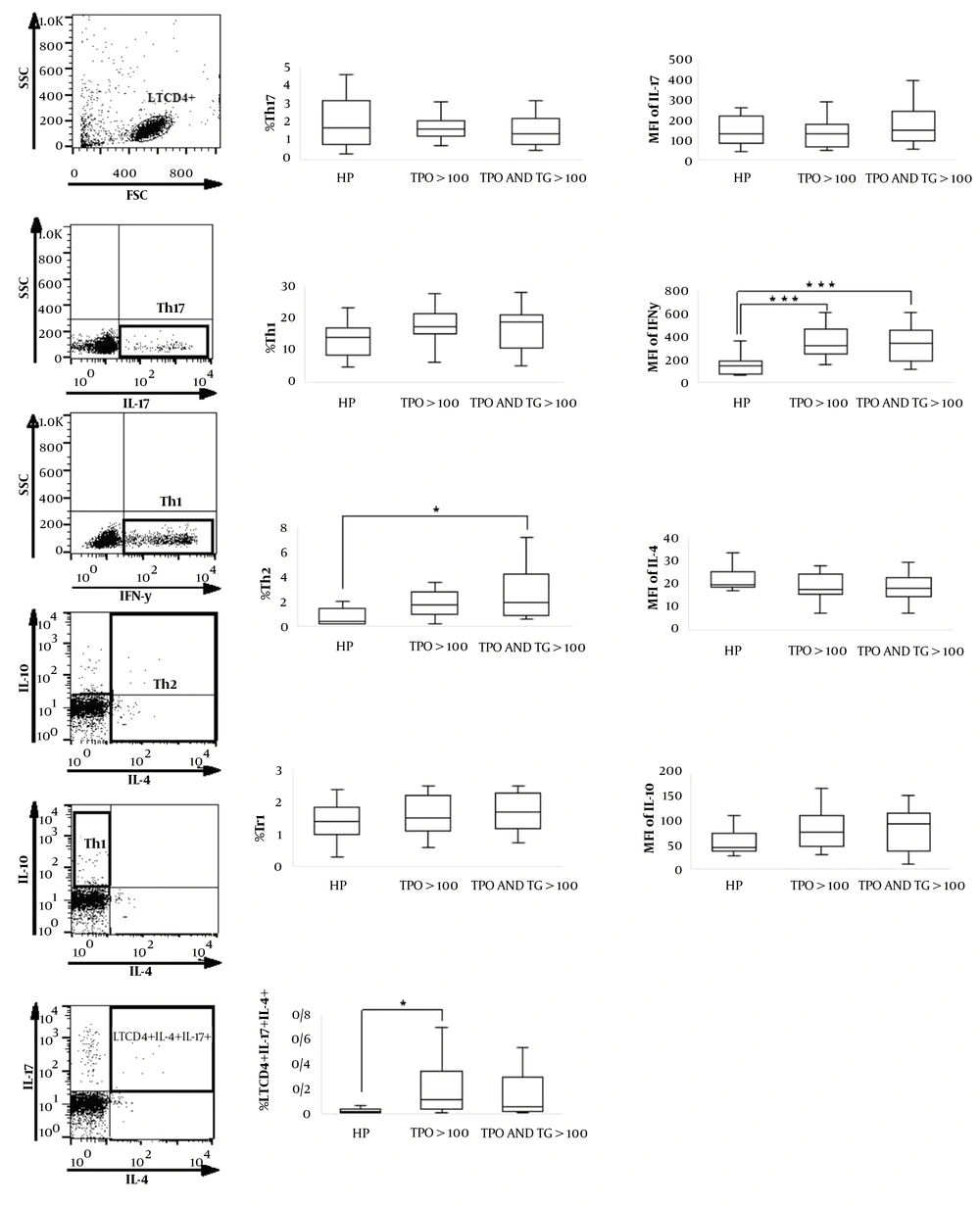
Appropriate isotype controls include mouse IgG1 K isotype control FITC, mouse IgG1 K isotype control APC, Mouse IgG1 K isotype control PerCP-Cyanine5.5, and Rat IgG1 K isotype control PE were used for gating and confirming the specificity of antibodies. Flow cytometry analysis was performed on FACSCalibur flow cytometer (BD Bioscience) by accumulating up to 20,000 events per tube. The data were analyzed using FlowJo software (Tree Star, Ashland, OR, USA). Th1, Th2, Tr1, and Th17 cells were identified as IFN-γ–secreting CD4+ T cells, IL-4–secreting CD4+T cells, CD4+ IL-10+IL-4- T cells, and IL-17–secreting CD4+ T cells, respectively (Figure 1). Data were analyzed first by gating the lymphocyte population as defined by forward and side scatters. Then selected T cells were entered into a 2-dimensional dot plot. Flow cytometric analysis was performed for all patients before and after a 3-month vitamin D supplementation. Mean fluorescent intensity (MFI) was also calculated by the mean of the population of interest in FlowJo software.
Evaluation of CD4+ T cells frequency in women with Hashimoto's thyroiditis. For intracellular staining, CD4+ T cells were stimulated with PMA and ionomycin for 6 h and then the cells were washed, fixed, permeabilized, and incubated with monoclonal antibodies. Finally, the frequencies of Th1, Th2, Th17, LTCD4+IL-17+IL-4+, and Tr1 and MFI of their related cytokines were analyzed by flow cytometry. Th1, Th2, Tr1 and Th17 cells were identified as IFN-γ secreting CD4+ T cells, IL-4 secreting CD4+ T cells, CD4+ IL-10+IL-4- T cells and IL-17 secreting CD4+ T cells, respectively. *P < 0.05, **P < 0.01, ***P < 0.001 within groups.
2.4. Measurement of Thyroid-Stimulating Hormone Autoantibodies
Plasma obtained from patients’ blood and samples were aliquoted and stored at -70°C pending further analysis. Plasma concentrations of thyroid-stimulating hormone (TSH), anti-TPO, and anti-TG were obtained by ELISA kits (Monobind, Inc, USA).
2.5. Statistical Analysis
Analysis of t-test was used to compare the frequency of Th17, Th1, Th2, and Tr1 cells and MFI of their cytokines in all study groups.
All statistical tests were two-sided, and P-value of less than 0.05 was considered statistically significant. All data analyses were performed by SPSS statistical software (version 22).
3. Results
In this study, the frequency of Th2 cells was not as high in the group 1 (anti-TPO > 100) as it was in the group 2 (anti-TPO > 100 and anti-TG > 100) in comparison to the group 3 (anti-TPO < 100 and anti-TG < 100). Only the difference of Th2 cells between the groups 3 and 2 was significant (P = 0.022; Table 2 and Figure 1). The frequency of LTCD4+IL-4+IL-17+ cells in the group 1 (anti-TPO > 100) was significantly more than group 3 (healthy persons) (P = 0.027); however, this difference between groups 3 and 2 was not found significant (P = 0.126; Table 2 and Figure 1).
| Variables | Anti-TPO > 100 and Anti-TG > 100 (n = 17) | Anti-TPO > 100 (n = 16) | Healthy Women (n = 13) | P-Value |
|---|---|---|---|---|
| Th1, % | 18.9 (10.8 - 21.0) | 17.3 (15.2 - 21.5) | 14.3 (8.8 - 17.2) | 0.135 |
| Th2, % | 1.8 (0.73 - 4.0) | 1.5 (0.76 - 3.3) | 0.49 (0.31 - 1.5) | 0.023 b |
| Th17, % | 1.4 (0.87 - 2.2) | 1.6 (1.2 - 2.1) | 1.7 (0.81 - 3.2) | 0.761 |
| Tr1, % | 1.7 (1.1 - 2.3) | 1.5 (1.1 - 2.2) | 1.4 (1.0 - 1.8) | 0.460 |
| LTCD4+IL-17+IL-4+ | 0.06 (0.01 - 0.4) | 0.12 (0.04 - 0.35) | 0.02 (0.007 - 0.05) | 0.026 b |
| LTCD4+ IFN-γ +IL-10+ | 0.94 (0.45 - 1.4) | 0.81 (0.52 - 1.4) | 0.66 (0.4 - 0.8) | 0.474 |
| LTCD4+ IFN-γ +IL-17+ | 0.21 (0.14 - 0.48) | 0.35 (0.24 - 0.45) | 0.33 (0.18 - 0.47) | 0.493 |
| LTCD4+IL-17+IL-10+ | 0.13 (0.08 - 0.25) | 0.22 (0.12 - 0.28) | 0.18 (0.13 - 0.28) | 0.293 |
| IFN-γ (MFI) | 333.00 (183.00 - 442.00) | 318.00 (245.00 - 455.00) | 142.00 (73.00 - 185.00) | 0.000 b |
| IL-10 (MFI) | 84.00 (40.00 - 109.00) | 78.00 (52.00 - 112.00) | 48.00 (40.00 - 73.00) | 0.090 |
| IL-4 (MFI) | 18.00 (14.00 - 22.00) | 17.00 (15.00 - 24.00) | 19.00 (18.00 - 25.00) | 0.098 |
| IL-17 (MFI) | 148.00 (95.00 - 242.00) | 134.00 (69.00 - 175.00) | 132.00 (85.00 - 218.00) | 0.331 |
Abbreviations: MFI, mean fluorescent intensity; SEM, standard error of mean.
a Values are expressed as median (Q1 – Q3).
b Mean value was significantly different compared to the control group (Healthy women).
No significant difference was observed in terms of the frequency of Th1 in three groups, but the difference of IFN-γ MFI was significant between the group 3 and group 1 (P = 0.001) as well as group 2 and group 1 (P = 0.001; Table 2 and Figure 1). Th2 cells frequency and MFI of IFN-γ in group 2 (anti-TPO > 100 and anti-TG > 100) were higher than patients in group 1 (anti-TPO > 100) (Table 2) and (Figure 1). No significant differences were found in terms of the frequency of Th17 and Tr1 cells and in MFI of IL-17 and IL-10 (Table 2 and Figure 1).
4. Discussion
Many studies have been done to investigate the role of immune cells involved in the pathogenesis of Hashimoto’s thyroiditis disease. Both humoral (autoantibodies) and cellular immunity have been shown to play important roles in the pathogenesis of this disease. Th1 cells are predominant cells in the tissues of the thyroid in patients with HT. In most studies, it has been shown that these cells are significantly increased in patients with HT. Th1 cells cause thyroiditis and thyroid gland damage by activation of macrophages and cytotoxic lymphocytes, which destroy thyroid follicular cells. Furthermore, in HT, cytokine stimulation from antigen-presenting cells and Th1 cells by inducing the expression of functional Fas receptor and Fas ligand on thyroid follicular cells may cause self-apoptosis (7).
In our study, it was also found that the expression of IFN-γ in these cells was significantly higher in patients with HT than in normal individuals. It seems that this cytokine has an important effect on the pathogenesis of this disease. In order to control these diseases reducing the expression of IFN-γ or avoid recruiting IFN-γ producing cells to thyroid tissue may be effective.
IFN-γ and TNF-β secreted by recruited Th1 cells stimulate CXCL10, which causes recruits Th1 cells expressing CXCR3. Therefore, creating an amplification feedback loop, initiating and perpetuating the autoimmune process (6). CXCR3 antagonist or CXCL10 blocking by avoiding the recruitment of Th1 cells may be helpful in the treatment of HT.
Our results showed an increased percentage of Th2 cells in patients with HT. A similar result was seen in another study conducted by Yanying Guo. They found that the proportion of Th2 cells in CD4+ T cells was significantly higher in patients with HT than those with euthyroid controls (15).
It was thought that the Th1 pattern of the immune response characteristic of cellular immunity was dominant in HT and humoral immunity was driven by Th2 cytokines lead to graves disease (GD). However, recently this assumption has been outweighed by new data showing that both HT and GD are Th1- and Th2-associated diseases (6-8). In a study done by Rebuffat et al., it was found that anti-TPO antibodies contribute to thyroid destruction through complement activation and antibody-dependent cell-mediated cytotoxicity (ADCC), indicating HT is also a Th2-associated disease (16).
The Th17 cell population did not increase in our study. Although some studies showed that Th17 cells did not change in HT, most studies indicated an increase in this cell population in patients with HT (17).
It has been shown that in autoimmune disease, a failure of tolerance mechanisms can be observed. Tr1 cells are one of the most important immune regulatory cells (18). In most autoimmune diseases, the lack of performance or reduction in the population of these cells has been observed (19, 20); however, in our study, there was no significant difference in the population of these cells between patients with HT and normal persons.
In a study done by Nielsen et al., it was found that in patients with HT, not only the Tr1 cell population was not decreased, but also the number of these cells and IL-10 expression under the influence of TG were increased (21).
The results of another study on patients with autoimmune thyroid disease (AITD) showed that regulatory T lymphocytes increased in these patients, but these lymphocytes exhibit a poor immunosuppressive function. It is very likely that increase of regulatory T lymphocytes acts as a compensatory mechanism to control the ongoing autoimmune process; however, because of their poor immunosuppressive function, they cannot perform their function (22).
In fact, the main problem is not the lack of these cells or the reduction of IL-10 expression, but it may be dysfunctional IL-10, which makes it incapable to effectively downregulate the ongoing autoimmune process and inflammatory phenomenon. Therefore, it is advisable to work on the signaling pathways of this cytokine in this disease. Possibly by finding out the exact defective signaling pathway, the appropriate remedy will be discovered.
4.1. Conclusion
The results of the present study showed that Th2 cells increased in women with HT, while in previous studies, HT had been described as a Th1-associated disease. Th2 cells frequency and the expression of IFN-γ in the patients who had both anti-TPO and anti-TG more than 100 IU/mL were higher than patients who had just anti-TPO more than 100 IU/mL. These results indicate that by increasing the severity of disease, the expression of IFN-γ and frequency of Th2 are elevated.
Although no significant differences were not observed in the frequency of Th17 and Tr1 cells or in MFI of IL-17 and IL-10 in comparison to normal individuals, targeting these cells frequency or the expression of their cytokines in the treatment of this disease needs more investigations. According to these findings, reducing the frequency of Th2 or the expression of IFN-γ may be effective in controlling this disease progression. However, more studies are necessary to evaluate the exact pathogenetic mechanisms of HT disease.