1. Background
Diabetes mellitus (DM) is a prevalent chronic disease worldwide with an increasing trend in its incidence (1). It is considered a severe public health issue in all countries due to its economic burden and its negative effect on the quality of life of the patients caused by its complications, such as retinopathy, foot ulcer, nephropathy, neuropathy, and coronary artery diseases, and comorbidities, such as obesity, hypertension, dyslipidemia, depression, and arthritis (2). The DM is even a more important issue in women, not only due to the higher risk of cardiac complications (3) but also due to the higher risk of complications during pregnancy for the mother (e.g., preeclampsia, cesarean section, and DM-related complications), the fetus (e.g., altered organogenesis and growth), and the offspring (e.g., higher risk of childhood adiposity, cardiovascular diseases, and DM) (4, 5).
Congenital heart disease (CHD) is an important complication of gestational diabetes mellitus (GDM) and pregestational DM with a 4-fold increased risk, irrespective of the type of antidiabetic treatment received (6, 7). Due to the linear relationship between the incidence of fetal CHD and high maternal glucose levels before (8) and during pregnancy (9), close monitoring to control glucose levels has been suggested for all mothers with GDM or pregestational DM (10), which has also been shown to reduce the risk of future cardiovascular complications in the mother (11). However, the universal screening of GDM is performed within 24 - 28 weeks of gestation; nevertheless, the cardiac development occurs in the first trimester and is almost completed by 6 weeks of gestation (12); therefore, high glucose levels can induce negative effects on the fetal heart before the diagnosis of GDM (13). Accordingly, there is a need for the accurate diagnosis and assessment of cardiac anomalies of the fetus, for which fetal echocardiography has been suggested as a powerful tool (14, 15). Furthermore, CHD is not the only cardiac disease in the fetuses of mothers with GDM or pregestational DM. Other cardiac diseases, such as hypertrophic cardiomyopathy (HCM) and decreased cardiac performance (due to functional abnormalities), are also associated with the mother’s DM, which can only be detected by complete fetal echocardiography (16, 17).
Fetal cardiac function assessment by Doppler fetal echocardiography (indicating cardiac output and systolic and diastolic dysfunction) can provide important information about cardiac abnormalities, increases the neonates’ survival rate, and reduces complications, mortality, and morbidity (18, 19).
2. Objectives
According to the significance of cardiac anomalies in the fetuses of diabetic mothers, this study aimed to determine the effect of maternal DM, both GDM, and pregestational DM, on fetal cardiac function in the second and third trimesters by comparing the parameters of fetal Doppler echocardiography between mothers with and without DM and their association with glycemic control during pregnancy.
3. Methods
In the current case-control observational study, women with singleton pregnancy in their second or third trimester (18 - 40 or 28 - 40 weeks of gestation) underwent a single additional cardiac scan as they had attended the ultrasound departments for routine prenatal or anomaly ultrasound examination at Mahdiyeh and Shohada hospitals, Tehran, Iran, within March 2020 to February 2021 and were enrolled for participating in this study.
The study protocol was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (IR.SBMU.MSP.REC.1399.545). Before enrollment, the researcher explained the design of the study to the eligible mothers and asked them to read and sign the written informed consent. The selection of patients to complete the questionnaire and then undergo an ultrasound in both groups was completely blind and random.
Mothers who gave consent for participation were enrolled in the study using the census method. Mothers with a positive history of overt DM (pregestational) or GDM in the current pregnancy were considered the case group, and mothers without GDM or DM were considered the control group. The inclusion criteria were women with singleton pregnancies in the second trimester (18 - 28 weeks of gestation) or the third trimester (28 - 40 weeks of gestation). The exclusion criteria were multifetal pregnancy, hypertension (pregnancy-induced or essential), intrauterine growth retardation (defined as weight for gestation less than 10th percentile), and polyhydramnios in the study.
According to the above-mentioned criteria, a total of 100 mothers were enrolled, 50 subjects in the case and 50 subjects in the control group. All mothers underwent aneuploidy screening test in the first trimester, three of whom were identified as high-risk combined tests, and one had increased nuchal translucency and underwent amniocentesis. All mothers had normal results of anomaly scans within 18 - 20 weeks of gestation.
The physician collected the mothers’ information by taking a medical history from the mothers, including age, parity, body mass index (BMI), type of antidiabetic medication, use of drugs, and underlying medical diseases. A previous result of their fasting blood sample was evaluated. The mothers were categorized into the case or control groups according to their fasting blood sugar (FBS) or 2-hour oral glucose tolerance test (OGTT). The analysis results were obtained from all cases to differentiate between diabetic and control cases, considering the cut-off levels of < 92 mg/dL for FBS and < 153 mg/dL for OGTT.
Furthermore, mothers in the case group were categorized into poor control DM and normal glycemic level (good control DM) according to the American Diabetes Association and American College of Obstetricians and Gynecologists, considering the target glucose concentrations at FBS < 95 mg/dL, 1-hour postprandial blood glucose concentrations < 140 mg/dL, and 2-hour postprandial glucose concentrations < 120 mg/dL. The researchers initiated insulin therapy (or increased doses of glycemic control medications) when one-third of fasting or postprandial glucose levels exceeded the target in a given week.
The imaging protocol performed for all mothers consisted of ultrasound, performed by one fellowship of perinatology using machine GE Vivid E8 Ultrasound System, which included the following steps:
(1) Fetal echocardiography was performed using the echocardiographic device, as described by Alfred Abuhamad as cited in Carvalho (20), for the measurement of the end-diastolic interventricular septal thickness (IVST) and myocardial free walls (inferior to atrioventricular valves), including right ventricular wall thickness (RVWT), left ventricular wall thickness (LVWT), recorded in lateral subcostal, apical, or basal four-chamber view (based on the fetal position at the time of scan).
(2) Doppler waveform was performed to measure the left myocardial performance index (MPI), as described by Hernandez-Andrade et al. (21). After obtaining a five-chamber view of the fetal heart, the ascending aorta was aligned with an angle of insonation at < 20°, and after visibility of the mitral and aortic valves, the Doppler sample gate was opened to about 3 - 4 mm and applied over leaflets of mitral and aortic valves to identify valve clicks along the Doppler waveforms after minimizing noise and artifacts. The procedure was repeated three times to enhance reproducibility.
The pulsed Doppler sample volume was placed on the inner wall of the ventricular septum above the mitral valve and below the aortic valve in the four-chamber view with a basal or an apical projection allowing simultaneous inflow and outflow display from the left ventricle. Then, time periods for the left MPI were measured as follows:
The time period from the mitral valve closure clicks to the opening of the aortic valve click (in milliseconds) was considered isovolumic contraction time (IVCT).
The time period from the aortic valve closure clicks to the opening of the mitral valve click (in milliseconds) was considered isovolumic relaxation time (IVRT).
The time period from the opening to the closure of the aortic valve click (in milliseconds) was considered ejection time.
Fetal left ventricular MPI was calculated by the following equation:
(3) Pulsed Doppler interrogation of atrioventricular valves was performed in the standard apical four-chamber view with sampling volume from distal to tip of the valve. The mean peak values of three early diastolic waves (E) and of three late diastolic or atrial filling waves (A) were recorded, indicating tricuspid and mitral inflow velocities (depicting passive and active ventricular filling); the mean ratio between the peak velocities of the E and A waves in each position (E/A ratio) was determined at the mitral and tricuspid valves, indicating diastolic function.
According to the reported mean duration of IVRT as 34 milliseconds with a range of 26 - 41 milliseconds (20), IVRT values > 41 milliseconds were considered prolonged. According to the same reference, the normal range of fetal left ventricular MPI was considered within 0.26 - 0.44 (mean: 0.36) and MPI > 0.44 as prolonged (20). In addition, the cut-off values of IVST and RVWT were considered at 4 mm and LVWT at 4.9 mm (20).
Finally, the intraobserver reproducibility of the MPI, IVST, E/A ratios, interventricular thickness, and free wall ventricle thickness were analyzed in 100 women within 18 - 40 weeks of gestation.
3.1. Statistical Analysis
The baseline characteristics of the participants are presented as mean ± standard deviation. The t-test was used to compare two groups of diabetic and nondiabetic mothers. The machine learning method was used to extract important predictors of fetal myocardial function in the case and control groups. Three types of decision tree (DT) algorithms, including classification and regression tree (CART) algorithms, chi-square automatic interaction detection (CHAID), and quick, unbiased, efficient, statistical tree (QUEST), were used for analysis (22). The accuracy of the models is attached in Appendix 1.
The target variable belonged to case and control groups defined by diabetes (i.e., diabetic and nondiabetic). The input variables (predictors) included the fetal left ventricular MPI, mitral valve (MV) E/A ratio, tricuspid valve (TV) E/A ratio, RVWT, LVWT, and IVST. The total data were divided into 70% (training data) and 30% (test data) for developing and testing DT algorithms, respectively. The performance of DT models was measured on the test data with accuracy, sensitivity, and specificity. IBM SPSS Modeler software (version 18) and Payton software (version 1) were used for statistical and DT analysis. A P-value less than 0.05 was considered statistically significant.
4. Results
A total of 100 mothers were enrolled with a mean age of 29.59 ± 5.52 years, mean gestational age (at the first examination) of 23 ± 3.4 weeks (median: 22 weeks), and mean BMI of 26.36 ± 0.41 kg/m2. In the case group (n = 50), 40 women had GDM (80%), among whom 22 subjects controlled their DM by nutrition therapy and 18 subjects used antidiabetic drugs (metformin or insulin); moreover, 10 patients had overt DM (20%), who used insulin. In the case group, 22 mothers had poor glycemic control.
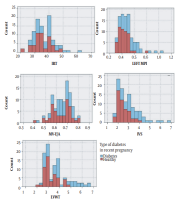
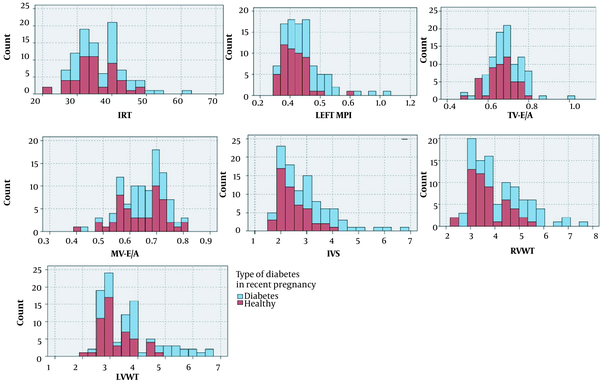
As shown in Figure 1A, the frequency of prolonged IVRT (> 41 milliseconds) was significantly higher in the case group than in the control group. The mean IVRT was also different between mothers with poor and good glycemic control (36.57 and 37.16, respectively; P < 0.05).
Distribution of echocardiographic parameters in cases and controls. Abbreviations: IRT, isovolumic relaxation time; MPI, myocardial performance index; TV, tricuspid valve; MV, mitral valve; IVS, interventricular septum; RVWT, right ventricular wall thickness; LVWT, right ventricular wall thickness.
The mean values of fetal left ventricular MPI were 0.53 ± 0.15 and 0.43 ± 0.09 in the case and control groups, respectively, with no statistically significant difference (P < 0.05). In the case group, the mean values of the left MPI were 0.57 ± 0.18 and 0.49 ± 0.12 in mothers with poor and good glycemic control, respectively (P = 0.12; Table 1). Additionally, the mean MPI was not different between the two subgroups of the case group (overt DM and GDM: 0.58 and 0.51, respectively; P > 0.05), neither among the three subgroups of the case group (mothers with GDM under nutrition therapy, under antidiabetic drug, and overt DM: 0.49, 0.54, and 0.58, respectively; P = 0.08). As shown in Figure 1B, the frequency of the left MPI within the normal range of MPI was higher in the control group, and the frequency of MPI > 0.44 was higher in the case group.
| Glucose Control | N | Mean ± SD |
|---|---|---|
| Good | 28 | 0.49 ± 0.11 |
| Poor | 22 | 0.57 ± 0.18 |
Mean Left Myocardial Performance Index in Diabetic Group according to Glucose Control
The mean values of IVST in the case and control groups were 3.3 ± 1.11 and 2.49 ± 0.55 mm, respectively (P < 0.05). The IVST > 4 mm was only observed in the case group (Figure 1E). The mean values of RVWT were 4.47 ± 1.2 and 3.6 ± 0.72 mm in the case and control groups, and the mean values of LVWT were 4.02 ± 1.19 and 3.19 ± 0.63, respectively, with a statistically significant difference (P < 0.05). The RVWT > 5.8 mm and LVWT > 4.9 mm were only observed in the case group (Figure 1F and G). A significant statistical difference was observed regarding RVWT and LVWT between diabetic and control groups (P < 0.05; Table 2).
| Variables | Diabetes | Healthy | P-Value |
|---|---|---|---|
| Left MPI | 0.53 ± 0.15 | 0.43 ± 0.09 | < 0.05 |
| IVST | 3.3 ± 1.11 | 2.49 ± 0.55 | < 0.05 |
| RVWT | 4.47 ± 1.2 | 3.6 ± 0.72 | < 0.05 |
| LVWT | 4.02 ± 1.19 | 3.19 ± 0.63 | < 0.05 |
Comparison of Variables in Fetuses of Diabetic and Nondiabetic Mothers a
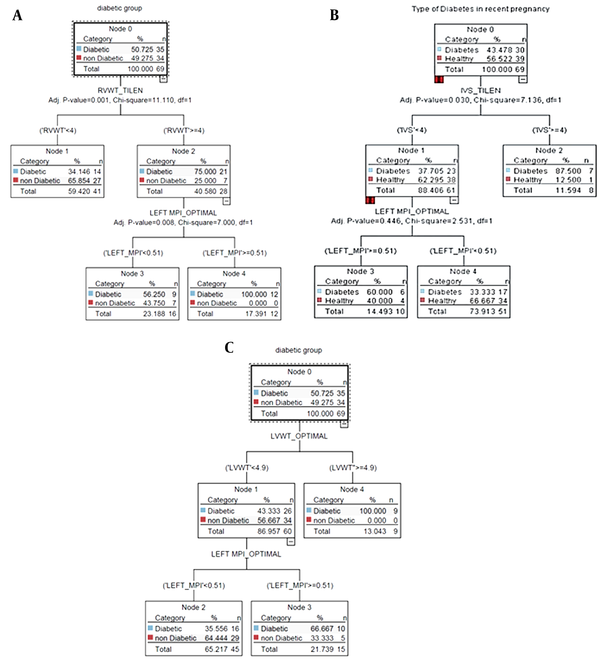
According to Figure 2A (CART model), all patients who had concurrent RVWT ≥ 4 mm and MPI ≥ 0.5 belonged to the diabetic group. The sensitivity of diabetes classes in the CART model was equal to 97%, which was higher than in other models (Table 3).
| Diabetes | Healthy | |
|---|---|---|
| Partition: 1- Training | ||
| Diabetes | 34 | 1 |
| Healthy | 29 | 5 |
| Partition: 2- Testing | ||
| Diabetes | 13 | 2 |
| Healthy | 13 | 3 |
Confusion Matrix for the Classification and Regression Tree Algorithm
In 88% of the participants, the fetal interventricular septum (IVS) of the fetal heart was less than 4 mm, which was within the normal range. Furthermore, 14% of patients with normal fetal IVS had an MPI above 0.5 simultaneously, which was within the abnormal range. It was noteworthy that 60% of the above-mentioned individuals (with abnormal MPI and normal IVS) belonged to the group of diabetic mothers (Figure 2B, CHAID). Of all participants, 60 mothers (86%) had LVWT ≤ 4.9 mm, among whom 66.6% and 33.33% of the case and control groups had a left MPI ≥ 0.5, respectively (Figure 2C, QUEST).
5. Discussion
In the present study, the comparison of diabetic mothers (80% GDM and 20% overt DM) to the control group (nondiabetic mothers) showed a significant change in several parameters of fetal Doppler echocardiographic parameters. The current study evaluated fetal cardiac function by measuring the E/A ratio (the evaluation of diastolic cardiac function), fetal left ventricular MPI (systolic and diastolic heart function), and ventricular wall thickness (IVST, LVWT, and RVWT) (23). The fetal cardiac cycle involves five distinct phases, namely (1) early diastole, (2) atrial contraction, (3) isovolumetric contraction, (4) ejection phase, and (5) isovolumetric relaxation (24). Therefore, the evaluation of fetal cardiac function should include all phases for an accurate diagnosis, as performed in the present study.
The results of this study indicated a higher mean fetal left ventricular MPI and a higher frequency of MPI > 0.44 in diabetic mothers, which showed increased MPI, denoting the maximum normal value of 0.43, suggested by Hernandez-Andrade et al. within 19 - 39 weeks of gestation (21). Increased MPI in the fetuses of diabetic mothers indicates damage to the myocardium due to the negative effects of high glucose levels (25). The results of the present study are consistent with the results of previous studies, comparing the MPI values of fetuses of diabetic mothers and healthy controls (26-28). As indicated, fetal left ventricular MPI was a significant predictor of adverse neonatal outcomes, which shows the importance of MPI in screening the fetuses of diabetic mothers (26, 27). The MPI evaluates both systolic and diastolic dysfunction by the two components of IVCT and IVRT, respectively (29). The findings of the present study showed a significantly higher frequency of prolonged IVRT (> 41 milliseconds) in the case group, which suggested the presence of diastolic dysfunction in the fetuses of diabetic mothers. The aforementioned results are consistent with the results reported by Atiq et al., which indicated prolonged IVRT in diabetic mothers in the second trimester with a significant difference, compared to the control group (30). In addition, mothers with good glycemic control had a significantly longer IVRT, and there was no difference in the mean MPI of diabetic mothers with poor or good glycemic control, although both were higher than the maximum level; these results indicated the significance of MPI in evaluating the fetal cardiac function, irrespective of the glycemic control in the mother.
Another cardiac parameter given special attention is the E/A ratio, which reflects the cardiac preload. The E changes in different weeks of gestation by the development of cardiac ventricles; however, the A remains more constant (21). The results of the present study showed an E/A ratio of < 1 in both left and right heart in the case and control groups, which suggests that the atrial contraction component of the cardiac cycle contributed to the majority of blood in diastole, rather than the negative pressure during relaxation, possibly due to chronic hypoxia, cardiac overload (21), or intrauterine growth restriction (31). However, due to the variable ratio in different gestational weeks and the lack of a standard reference value for E/A, it is suggested to evaluate all the cardiac parameters together for a thorough fetal heart assessment.
For the evaluation of fetal myocardial hypertrophy, IVST, RVWT, and LVWT are evaluated, and the results of the present study showed higher mean values of all cardiac walls in the case group than in the control group; nevertheless, IVST > 4 mm, RVWT > 5.8 mm, and LVWT > 4.9 mm were only observed in the case group, which suggested diastolic dysfunction and the possibility of HCM, the confirmation of which requires the consideration of all cardiac parameters together and might require further assessments (32). The results of the present study are consistent with the results of a study by Raafat et al., which compared the cardiac wall thickness of 40 pregnant mothers with pregestational DM (types I and II), metabolically controlled during pregnancy, to healthy controls (28). Other studies have also shown that the fetuses of diabetic mothers have a thicker IVST than the control group (33).
As the cardiac parameters have to be justified in junction with each other, the current study evaluated the associations between the cardiac parameters, and the results showed that among 88% of all pregnant mothers with MPI ≥ 0.51, only 14% had IVST ≥ 4 mm, all belonging to the diabetic group, which suggested the association of abnormal cardiac parameters. However, the presence of normal LVWT and RVWT in the fetuses of diabetic mothers with increased MPI suggests that MPI disorder can occur early and independent of fetal cardiac hypertrophy, which is in line with the results of a study by Mohsin et al. (34); therefore, it is recommended to perform a complete cardiac function test for diabetic mothers and other high-risk fetuses to recognize early and subtle changes in fetuses in order to improve management and outcome (18, 19).
The limitations of the present study were the small sample size and nonrandomized inclusion of participants in the study and the two groups.
5.1. Conclusions
Prenatal complete echocardiography in the second and third trimesters is a helpful tool for the assessment of the fetuses of diabetic mothers (overt and GDM). Due to the high prevalence of HCM and cardiac function impairment in these fetuses and the relative ease of access and performance of echocardiography, it is recommended to include this test in the routine prenatal examination of all diabetic mothers. As the results of the present study suggested, the functional cardiac abnormalities of the fetuses were not associated with glycemic control in the mothers and can occur in the fetuses of diabetic mothers without cardiac hypertrophy and structural disorders. Therefore, it is recommended to evaluate fetal cardiac function in all diabetic mothers, even those with good glucose control during pregnancy.