1. Introduction
Disseminated lymphoma may spread to any part of the body and secondary adrenal involvement is reported in 25% of autopsies (1, 2). But, primary adrenal lymphoma (PAL) is a rare entity, accounting for less than 1% of non-Hodgkin lymphomas and is more often of diffuse large B-cell type (3, 4). It predominantly affects the elderly males and adrenal insufficiency may be the presented symptom, especially in the ones with bilateral involvement, which may occur in two-thirds of the cases (3-5). The prognosis is poor and disease-free survival is not longer than 1 year in most reports, despite extensive treatment (3, 6). However, a high degree of suspicion of PAL is mandatory for timely diagnosis and management, particularly when evaluating patients with bilateral adrenal mass lesions.
2. Case Presentation
A 53-year-old male presented with 1 month history of abdominal pain, anorexia, and nausea. His medical history was positive for coronary artery disease and coronary artery bypass graft (CABG) surgery was performed on him, about 45 days prior to admission. He was relatively well when he developed abdominal pain accompanied by anorexia and nausea. The patient had sought medical attention for his abdominal discomfort, and bilateral large solid adrenal masses were detected in his abdominal ultrasonography. He also mentioned an unintentional weight loss and generalized darkening of the skin during this period.
He was admitted to the under study hospital with the impression of adrenal failure. Serum sample for basal cortisol and adrenocorticotropic hormone (ACTH) was taken in the basal state, and then, after giving 250 μg of synthetic ACTH, serum cortisol was measured again. He responded to administration of high doses of intravenous hydrocortisone with remission of symptoms. On admission, clinical examination revealed low blood pressure (90/60 mmHg), body temperature 37°C, the pulse rate 88 beat/minute, and respiratory rate of 18 breath/ minute. He looked well and was in no acute distress. There was generalized increased pigmentation of the skin. No Cushingoid appearance was detected and there was no palpable lymph node. Physical examination of lungs and heart were unremarkable and his abdomen was flat without palpable mass or hepatosplenomegaly. Laboratory investigations revealed normal liver function test, hemoglobin 11.1 g/dL, white blood cell 8.5 × 103, with normal differential count and platelet 200 × 103. Blood chemistries showed Na 125 mEq/L, K 5.2 mEq/L, blood urea nitrogen (BUN) 35 mg/dL, creatinine (Cr) 1.8 mg/dL, normal blood sugar, lactate dehydrogenase (LDH) 1277 U/L, erythrocyte sedimentation rate (ESR) 110 mm/hour, and normal lipase and amylase levels. The ultrasound of the abdomen revealed bilateral large solid adrenal masses without lymphadenopathy or organomegaly.
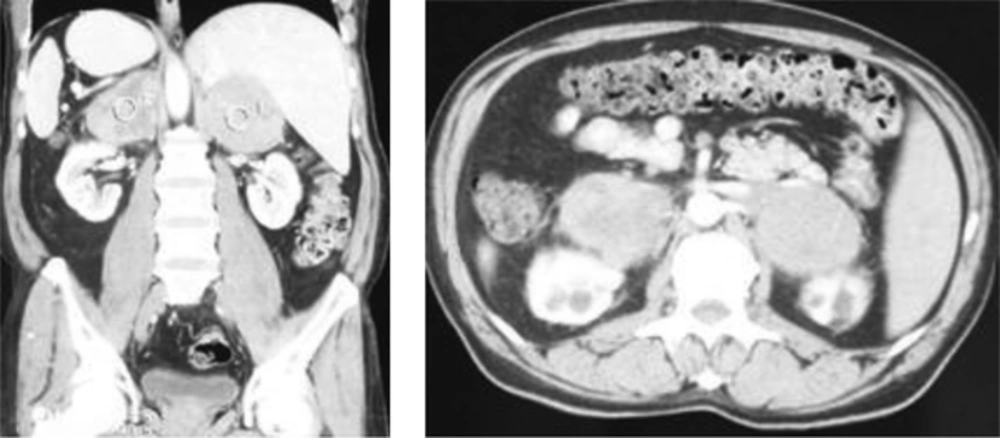
A contrast-enhanced computerized tomography (CT) scan of the abdomen with adrenal protocol demonstrated bilateral large heterogeneously enhancing adrenal lesions, 8.5 × 6.5 cm in the Rt. and 7.5 × 5.7 cm in the left adrenal, without calcification (Figure 1).
The masses were non-homogeneous and their precontrast Hounsfield score was 65 in the left and 73 in the right side. Also, there was no significant washout of the contrast agent and no other pathology was detected. Since adrenal metastasis from an unknown malignant disease was suspected, CT scan of the chest and pelvis was performed and no primary tumor was found. His upper endoscopy and colonoscopy also revealed no abnormal finding. Further biochemical evaluation showed normal alpha-fetoprotein (AFP), prostate-specific antigen (PSA), cancer antigen (CA) 19-9, U/mL, β-hCG, and CEA, and low dehydroepiandrosterone sulphate (DHEAS) levels and 24-hour urine metanephrines were 4.1 μg/day (0 - 350 μg/day).
Clinical suspicion of primary adrenal insufficiency was confirmed by an elevated baseline plasma ACTH of 99 pg/mL, a low plasma cortisol of 0.7 μg/dL (normal value of 12 - 25 μg/dL) and insufficient peak cortisol response of 1.9 μg/dL to ACTH stimulation (normal value of > 18 μg/dL). Bone marrow examination revealed no abnormality.
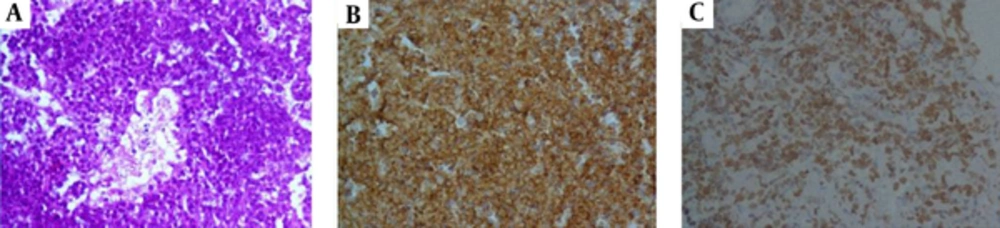
The patient was treated with high doses of intravenous hydrocortisone and rehydrated with sodium chloride infusion resulting in complete remission of symptoms and normalization of biochemistry. Subsequently, a CT-guided biopsy of the mass lesion was performed and microscopic examination of the material revealed diffuse large B-cell lymphoma.
Immunohistochemistry was positive for CD43, CD79, leukocyte common antigen (LCA), and Pax.5, but did not stain for CD3, CK, CK20, and cytokeratin (AE1/AE3). The proliferative fraction detected by Ki-67 was high and the diagnosis of B-cell lymphoma was confirmed (Figure 2).
Chemotherapy was started on him. At the end of the third cycle, there was a transient clinical improvement despite no change in size of the tumors. But, the patient worsened thereafter and died 5 months after starting the chemotherapy.
3. Discussion
The adrenal gland is a common site for metastases due to its rich blood supply. Indeed, metastatic tumors are the most common neoplasias that involve the adrenals and a variety of tumors including carcinomas of the lung, beast, kidney, and colon in addition to melanoma and lymphoma, can secondarily involve the adrenals (1, 2). Less than 5% of the adrenal incidentalomas are malignant and bilateral lesions may be observed in 10% to 15% of them (2, 5).
However, adrenal metastases are usually unilateral and diagnosed incidentally (7). But, bilateral adrenal masses are often secondary to metastasis (8). For example, in one reported series of 208 adrenal incidentalomas, 53% of the cases with proved adrenal metastases had bilateral disease (9).
When the masses are bilateral, the probable diagnoses include metastatic lesions, CAH, ACTH-dependent Cushing syndrome, infection, hemorrhage, lymphoma, and pheochromocytoma (1, 8). Primary adrenal lymphoma commonly presents as bilateral adrenal masses without any other extra-adrenal involvement. Furthermore, in contrast to adrenal metastases, which only infrequently cause hypoadrenalism, adrenal insufficiency is common in bilateral primary adrenal lymphoma, occurring in 50% to 70% of the cases (3, 5, 10).
However, due to non-specific clinical features and life-threatening consequences of adrenal crisis, immediate substitution therapy is recommended if primary adrenal insufficiency (PAI) is suspected (10, 11). The current study patient had bilateral adrenal masses in the process of work up for gastrointestinal symptoms and his clinical features and laboratory findings indicated the presence of primary adrenal insufficiency. Primary lymphoma of the adrenal gland is a rapidly progressive disease with poor prognosis. Advanced age, large tumor size, high serum levels of lactate dehydrogenase, and initial presentation with adrenal insufficiency are poor prognostic signs (1). However, the predominant histological subtype is high grade large B-cell lymphoma, but peripheral T-cell lymphoma is also reported in a minority of cases (6, 10, 12).
The etiology of PAL is unclear. Previously, it was supposed that the occurrence of PAI in such cases is the result of infiltration and destruction of the adrenal glands by lymphoid cells (10). But, Ellis and Read suggested that the human adrenal glands did not have lymphoid tissue. They subsequently concluded that the follicle center cell origin of PAL is suggestive of its development on a background of prior autoimmune adrenalitis, which is consistent with PAI in such patients (13). In this regard, Dutta et al., reported no correlation between adrenal insufficiency and the size of the tumor (14). Furthermore, it is proposed that Epstein-Barr virus infection, immune dysfunction, and mutations in the p53 and c-kit genes are also involved in the pathogenesis of PAL (8, 10, 15). However, most patients with PAL are asymptomatic until late in its course and lymphomatous involvement of the adrenals is usually discovered in postmortem examination (7). Therefore, it is recommended that all patients with bilateral adrenal lesions should be evaluated for adrenocortical hypo- and hyperfunction (10).
Gamelin et al., suggested that adrenal insufficiency might be underdiagnosed in patients with non-Hodgkin lymphoma. They found adrenal failure in four of 127 patients with non-Hodgkin lymphoma with bilateral adrenal involvement (16). Moreover, involvement of other sites outside the adrenals is rare at presentation, but later in the course of the disease, there is a propensity for generalized involvement of multiple organs such as the liver, stomach, and central nervous system (6, 10, 17, 18).
Imaging study with non-enhanced and then contrast-enhanced CT scans are primarily used to characterize the tumor. Density on CT scan is variable, but high Hounsfield score, low percentage of contrast wash-out, and large size of the tumor indicate its malignant nature. Further evaluation by histology is required to confirm the diagnosis, for which percutaneous ultrasound or CT-guided, and/or surgical biopsy are recommended (2, 5). Subsequent immunohistochemical studies have important implications to manage and determine the tumor prognosis (10).
At present, chemotherapy is the first-line treatment of PAL (5, 19), but the role of radiotherapy is unclear (6, 10). Furthermore, some authors recommended using laparoscopic adrenalectomy as adjuvant to chemotherapy for large masses. More recently, autologous peripheral blood stem cell transplantation is considered as a possible therapeutic option, especially in young patients (10, 11, 20). In this regard, some authors reported encouraging results, but its use should be individualized and favorable responses are achieved mainly in early stages of the disease. However, due to inadequate data and limited follow-up period in most cases, definite conclusion about the optimal treatment modality is not possible.
3.1. Conclusion
Primary adrenal lymphoma is extremely rare and should be considered in the differential diagnosis of bilateral adrenal masses. Moreover, adrenal failure is a common complication in patients with bilateral lesions. Since the development of Addisonian crisis can contribute to the patient’s morbidity and mortality, immediate glucocorticoid replacement therapy is recommended when adrenal insufficiency is suspected.