1. Context
Puberty is a critical period that begins between eight and 13 years old in girls (1, 2). It is accompanied by physiological changes in various organs (1, 2). Menarche is the last event of puberty and a turning point as the initiation of fertility (3, 4). Menstrual cycles are commonly irregular in the first years after menarche (5). The time interval between age at menarche and the onset of the regular menstrual cycle (menstrual cycle developmental trajectory) varies from several months to several years. Despite the introduction of several influential factors on this trajectory, there are still many unknowns (6, 7).
Close interaction between hypothalamic-pituitary-ovarian (HPO) axis hormones is fundamental for a regular and predictable ovulatory cycle (8). Several genetic and environmental factors from prenatal to later in life can affect the HPO axis. It has been shown that menstrual irregularity during adolescence may result from exposure to environmental stress during pregnancy and childhood (9). Later in life, several endocrine disorders, including polycystic ovary syndrome (PCOS), premature ovarian failure (POF), pituitary hypothalamic dysfunction, anorexia nervosa, thyroid dysfunction, and hyperprolactinemia, are often accompanied by menstrual irregularities (10).
The menstrual cycle pattern has a significant impact on the reproductive life of adolescents (11), and an irregular menstrual cycle threatens their physical and mental health (12, 13); whereas, this irregularity may be modifiable (14). Besides, long and irregular menstrual cycles are associated with premature mortality (15).
To the best of our knowledge, no review study has yet been conducted on the factors that influence menstrual cycle regularity in adolescents. Hence, this study aimed to summarize the factors related to adolescents' menstrual cycle developmental trajectory.
2. Evidence Acquisition
This narrative review aimed to determine the factors that influence the onset of adolescent menstrual cycle regulation. PubMed, Scopus, Google Scholar, and Web of Science electronic databases were searched for studies restricted to English papers published from 1970 to 2021 assessing the factors related to the onset of adolescent menstrual cycle regulation, using the following text words: "Puberty" OR "Adolescent" OR "Adolescence" OR "Menarche" OR "Menstruation" OR "Menstrual Cycle" OR "Ovulation" OR "Anovulation" AND "Obesity" OR "Pediatric Obesity" OR "Ethnicity" OR "Social Determinants of Health" OR" Air Pollution" OR "Geography" OR "Epigenesis" OR" Genetic" OR "Smoking" OR" Diabetes Mellitus" OR "Polycystic Ovary Syndrome" OR "Thyroid Diseases." We identified additional studies through a manual search of the bibliographic references of relevant articles and existing reviews. All relevant observational, experimental, and review studies investigating the pathophysiology of menstrual cycle developmental trajectory and its influential factors were included. We excluded non-human studies, commentaries, editorials, letters, proceedings, and studies that did not provide accurate and clear data or methods.
Menstrual cycle developmental trajectory is defined as a trajectory from anovulatory menstrual cycles to ovulatory menstrual cycles (7). Menstruation is defined as "cyclic bleeding from the uterine corpus between menarche and menopause" (16). Menarche was defined as the first menstrual bleeding during adolescence (17).
3. Results
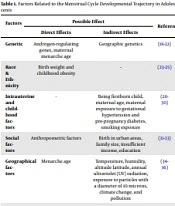
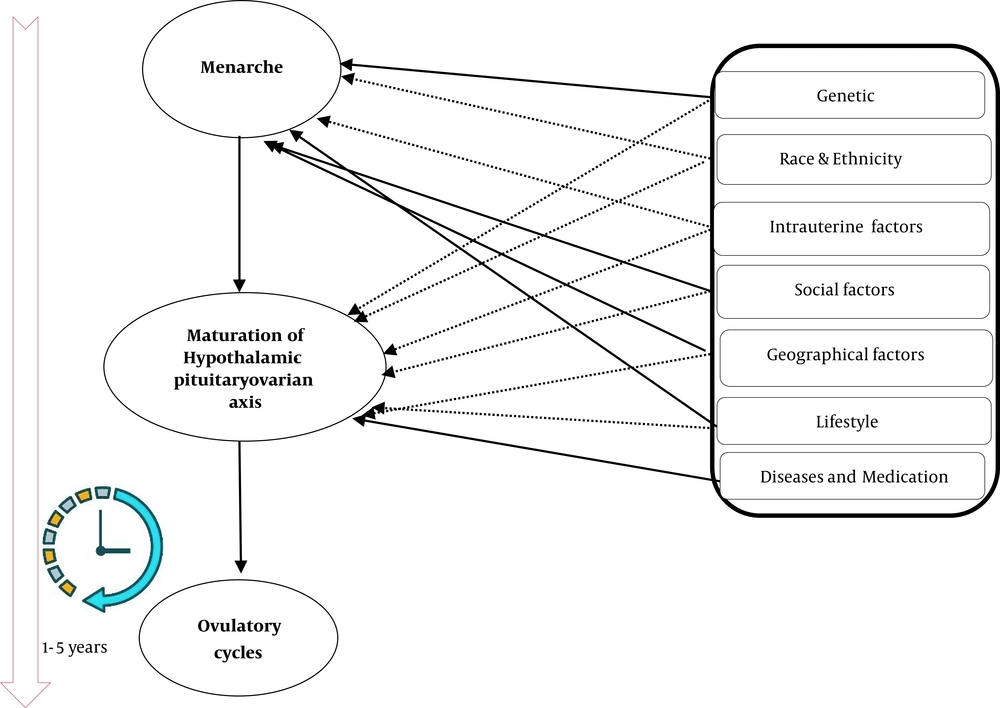
The titles, abstracts, and keywords were reviewed to exclude studies that did not fulfill the inclusion criteria in the initial screening stage. Of 240 records retrieved through searching databases, a total of 69 studies were included in this review. The factors influencing menstrual cycle developmental trajectory and their possible direct or indirect pathogenesis are summarized in Table 1 and Figure 1.
| Factors | Possible Effect | References | |
|---|---|---|---|
| Direct Effects | Indirect Effects | ||
| Genetic | Androgen-regulating genes, maternal menarche age | Geographic genetics | (18-22) |
| Race and ethnicity | Birth weight and childhood obesity | - | (23-25) |
| Intrauterine and childhood factors | - | Being firstborn child, maternal age, maternal exposure to gestational hypertension and pre-pregnancy diabetes, smoking exposure | (26-30) |
| Social factors | Anthropometric factors | Birth in urban areas, family size, insufficient income, education | (31-33) |
| Geographical factors | Menarche age | Temperature, humidity, altitude latitude, annual ultraviolet (UV) radiation, exposure to particles with a diameter of 10 microns, climate change, and pollution | (34-36) |
| Lifestyle | Anthropometric factors | Diet, physical activity, sleep, stress | (37-41) |
| Diseases and medication | Hormonal and metabolic disorders | - | (42-44) |
3.1. Neuroendocrine Development and Menstrual Cycle Regulation
Several observational studies revealed that the immaturity of the neuroendocrine system (HPO axis) and high serum androgens levels (45-47) are considered the two leading causes of the irregular menstrual cycle in adolescents. During puberty, the primary underlying mechanism of the cyclic secretion (sleep-wake cycle) of gonadotropin-releasing hormone (GnRH) remains unknown. The sleep-wake cycle of the GnRH pulse varies during puberty. The GnRH pulse is initially controlled by the state of sleep. During waking hours, the pulsatility of GnRH is sensitive to the neuronal signal inputs, which are sensitive to steroid hormones feedback. The wakening frequency of GnRH is regulated by progesterone and estradiol (48). A study among 23 postmenarchal adolescent girls described that an immature secretion of LH during sleep may lead to an irregular menstrual cycle (49). It has been shown that LH is higher in adolescents with irregular menstrual cycles than adolescents with regular menses (50, 51). The maturation of the HPO axis seems to be very complex and is controlled by other unknown mechanisms. Pena et al., in a cohort study among 40 healthy postmenarcheal girls, showed that sometimes ovulation occurred before the final maturation of the HPO axis. Therefore, they concluded that irregular menstrual cycles should not be considered the absence of ovulation in healthy adolescents (52). Moreover, a pilot study among 10 girls aged 11 - 13 years found that neuroendocrine axis maturation occurs during the first six months after menarche, and a delay in the onset of ovulatory cycles is due to the immaturity of the ovary (53). A recent cross-sectional study conducted by Sun et al. among 23 healthy girls demonstrated that almost three-quarters of the participants had at least one cycle with ovulation, but there was luteal insufficiency in most of them. Additionally, they observed immaturity in ovarian responses and FSH secretion (54). They highlighted the crucial role of concordant development of all elements of the hypothalamic-pituitary-ovarian axis in the maturation of the reproductive axis (54). Overall, for regular, predictable ovulatory menstrual cycles among adolescents, a tight, well-organized concordant function of all the three components of the HPO axis, including hypothalamus, pituitary, and ovary, is fundamental.
3.2. Age at Menarche
Menstruation is the monthly shedding of the functional layer of the uterine endometrial lining in the absence of fertilization (17). Numerous observational studies suggest that the onset of ovulatory menstrual cycles is strongly associated with menarche age (55-57). As shown in literature, during the transition in the second half of the 20th century, improvement in economic and social conditions significantly decreased age at menarche from 14 to 12 years old (58). A French (55) and a Japanese cohort study (56) showed that the interval between menarche and the onset of the regular menstrual cycle has increased despite the reduction in age at menarche. They found that a time interval of five years after menarche is needed for a regular menstrual cycle in most adolescent girls. This prolongation of the interval between menarche and the onset of the regular menstrual cycle and early menarche make immature mammary cells more susceptible to cancer (55).
Moreover, it may be associated with cardio-metabolic disturbances further in life or may influence the trend of their obesity indices (59, 60). A cohort study on 40 adolescents showed that although most adolescents had ovulation cycles during the first one to three postmenarchal years, their menstrual cycles were irregular (52). It can be concluded that the age of menstruation onset is one of the factors affecting this trajectory, and with a decrease in menarcheal age, this time interval is increased.
3.3. Genetic and Familial Factors
Genetic and familial factors are among the critical factors involved in puberty. Tu et al., in a longitudinal study, revealed that genetic diversity plays a vital role in the development of different stages of puberty (61). Several genes, including estrogen-metabolizing genes, CCR3 genes, and CYP17, contribute to menarche's age (18-20). Maternal age is considered the main factor influencing adolescents' menarche age and the timing of the onset of menstrual cycle regulation (59, 62). According to the results of two large studies, 23 to 57% of the difference in menarche age can be related to genetic and familial causes (21, 22). As mentioned earlier, elevated androgen levels influence the menstrual cycle regulation during adolescence. In recent years, the role of genetic factors in the development of PCOS, which can affect the onset of menstrual cycle regulation, has been explored. Recent molecular genetic studies have shown that androgen-regulating genes may play a role in developing hyperandrogenism (63).
The results of a cohort study on the Iranian population showed that the menarche age of girls was significantly correlated with the menarche age of their mothers (64). Moreover, an Italian population-based study demonstrated that geographic genetics (the birth location of parents) was associated with menarche age (65). Therefore, it seems that genetic and familial factors influence the timing of the onset of menstrual cycle regulation through neuroendocrine pathway development and genes associated with hyperandrogenism.
3.4. Race and Ethnicity
Ethnic and racial differences can play a role in the onset of menstrual cycle regulation and ovulation in adolescents (7). Harlow et al. reported that menstrual cycles longer than 45 days were 1.86 times more in European-American adolescents than in African-American adolescents (66). Also, the result of a cohort study demonstrated that adolescent girls of the Asian/Pacific Island races usually experience menarche earlier than their non-Hispanic white counterparts (67). Another study on different races also found that Korean girls experience menarche at a younger age than other races (Lisu, Kazakh); however, they reported that the better socioeconomic situation of Korean girls might partly explain this observed difference (23). Birth weight and childhood obesity are the other mechanisms that partly explain the difference in age of menarche and the onset of the regulatory cycle in various races. It has been shown that low birth weight and childhood obesity are more common in African-Americans than white women (24, 25). Racial differences in the developmental phases of puberty can also vary due to genetic factors (4). It can be concluded that race and ethnicity may influence the age of menarche and the onset of the regular menstrual cycle directly or indirectly through culture, lifestyle, socioeconomic situation, and obesity status.
3.5. Intrauterine and Childhood Factors
Exposure to environmental factors is one of the critical factors influencing the menarche age and, consequently, the time interval between menarche age and the onset of menstrual cycle regulation. Several studies revealed contradictory results on the effect of prenatal or childhood exposure to cigarettes at menarche (26-28). Cigarette smoking disrupts the placental blood flow (68) and may induce an adverse toxic effect on the ovaries (69). In a cohort study by Dossus et al., childhood exposure to cigarettes was associated with the increased interval between menarche and menstrual cycle regulation (29).
Birth length and weight are among the factors influencing the age of menarche. A longitudinal study in the Philippines showed that adolescents who weighed less than 3 kg at birth and those with birth length over 49 cm experienced menarche about six months earlier than adolescents who were thin and shorter at birth (30). Moreover, the result of the Sister Study among 33,501 women revealed that different exposures during the prenatal period that can affect sexual development and menarche include low birth weight, firstborn child, maternal age, gestational hypertension, and pre-pregnancy diabetes (70). Considering the adverse effect of early menarche on the interval between menarche and the onset of the regular menstrual cycle, these factors may indirectly affect the menstrual cycle developmental trajectory. Taken together, intrauterine and childhood factors have direct and indirect determinant roles in developing a regular menstrual cycle in adolescents.
3.6. Social Factors
Recently, Braveman and Gottlieb explained that social determinants of health play an essential role in public health (31). In this regard, more than two decades ago, a community-based study revealed the role of social factors in the evolution of sexual maturity and the age of menarche (30). A cohort study on 96,493 French women showed that social factors, including income index and birth in an urban area, were associated with a shorter interval between menarche and the onset of menstrual cycle regulation (29). Furthermore, in this study, family size (number of siblings) and place of residence were related to menarche age and the onset of menstrual cycle regulation (29). According to another study, the risk of menstrual irregularities was approximately 1.77 times higher in females with insufficient income and 1.46 times higher in adolescents who had inadequate contact with their parents (71). Besides, a study on Bosnian adolescent girls found that females who had a dysfunctional family structure (parental death, parental illness, divorce, etc.) experienced menarche earlier than others (32). The results of a cohort study on Iranian adolescent girls indicated that the age of menarche was affected by maternal education, meaning that an increase in maternal education was associated with reduced menarche age (33). It can be concluded that various social factors at the individual, family, and community levels can play a direct role in sexual development during puberty (72) or may indirectly exert their effect through their control on age at menarche onset.
3.7. Geographical Factors
Today, the impact of environmental factors on humans is indisputable. In general, geographical differences, including temperature, humidity, and altitude, can affect neuropeptide and neurotransmitter pathways related to puberty and menarche timing (34). In this regard, a study on female adolescents showed that girls born in summer experienced earlier menarche than females born in other seasons (35). Also, the results of a French cohort study showed that girls who lived at lower latitudes or in areas with higher annual ultraviolet (UV) radiation in spring and summer reached menarche three to four months earlier than others (73). Another cohort study in Hong Kong on 1,938 adolescent girls found that exposure to particles with a diameter of 10 microns, including nitrogen dioxide, sulfur dioxide, and nitric oxide, harmed puberty in girls (36). Climate change and pollution may affect the timing of menarche age. Living in mountainous areas and cold climates may affect menarche age in adolescents (74, 75). Climate change can affect menarche age by affecting food availability and increasing exposure to air toxins or pollutants (76). In general, geographical differences, including temperature, humidity, and altitude, can affect the pathways of neuropeptides and neurotransmitters related to menarche timing (34).
3.8. Lifestyle
Lifestyle can play an essential role in HPO axis development. Today, researchers believe that recent changes in diet and physical activity among adolescents contribute to increased menarche age and the onset of regular menstrual cycles (55, 59). It has been shown that pre-pubertal intake of milk, but not cheese and yogurt, may reduce the age at menarche (77). Sexual development and adaptation of the body to fertility can be affected by nutritional status in early life. Previous observational studies revealed that the consumption of animal proteins shortens the sexual development process, while it is prolonged by consuming plant-based proteins (37, 38). Contradictory findings were observed regarding the impact of infant nutrition, type, and duration of breastfeeding on the process of sexual development (39, 40). Anthropometric factors are essential in sexual development and puberty. In a study by Dossus et al., 835 Serbian adolescents in their sixth to 27th postmenarchal months were divided into females with regular and irregular menstrual cycles. Dossus et al. found that body silhouette at age eight and physical activity outside the school between the ages of eight and 15 were associated with the prolonged onset of menstrual cycle regulation (29). They also observed that adolescents with large body silhouettes experienced a 3.3-month longer interval between menarche and the commencement of a regular menstrual cycle compared to other females. The optimal body fat and stored energy are essential in forming the natural maturation and ovulation process (33, 78).
Another aspect of the lifestyle is sleep. In this regard, Nam et al. conducted a population-based study on 801 Korean adolescents (41). They observed that sleep duration less than five hours per day was associated with menstrual irregularities (41). Stress is one of the most important health disorders in today's world. Several studies demonstrated the adverse effects of various stressors during childhood and early adulthood on the interval between menarche and the onset of menstrual regularity (79, 80). Briefly, lifestyle factors have direct and indirect determinant roles in the menstrual cycle developmental trajectory.
3.9. Diseases and Medication
A normal menstrual cycle requires a normal production of insulin, thyroid, ovarian, adrenal, and hypothalamic hormones. Any disturbance in these pathways disrupts the normal process of the menstrual cycle (17). Therefore, endocrine disorders, including diabetes, thyroid disorder, and PCOS, can affect the menstrual cycle. Obesity and PCOS can increase androgens (81). A previous study on Brazilian teens showed that menstrual irregularities during the first postmenarchal year could be an indicator of PCOS in these females (42). The size of the ovaries increased with increasing age in adolescents with irregular menstrual cycles (82). This increase in ovarian size could be an indicator of the occurrence of PCOS in the future (82). Another study compared 56 adolescents with type 1 diabetes with 56 healthy adolescents and showed that amenorrhea (10.7% vs. 1.8%) and oligomenorrhea (58.9% vs. 19.6%) were more common in adolescents with diabetes than in healthy adolescents (43).
Researchers in a cross-sectional study found that the average menarche age was longer in adolescents with type 1 diabetes than in the non-diabetic group (79). Poor glycemic control is one of the mechanisms proposed for the menstrual irregularities and delays in regulating the menstrual cycle in adolescents with diabetes (83).
Thyroid disorders are among the endocrine disorders associated with menstrual cycle disorders during adolescence. In a study by Rajiwade et al. on Indian adolescents, 13.6% of the adolescents with menstrual disorders suffered from thyroid dysfunction (44). Additionally, in hormonal disease, medicines administered before menarche can affect sexual development in puberty (84). In conclusion, hormonal diseases and their medications may affect the pathways of neuropeptides and neurotransmitters related to puberty and menarche timing and may change the time interval between menarche and the age of the onset of regular ovulatory menstrual cycles.
3.10. Conclusions
Regular and predictable ovulatory cycles usually occur within five years after menarche (85). It is important to note that even in healthy girls with normal hormonal levels, the onset of the menstrual cycle and ovulation varies and depends on several known and unknown factors. Genetic, racial, ethnic, geographic, lifestyle, environmental, and socioeconomic factors can play essential roles in regulating menstrual cycles and the time interval between menarche and the age of the onset of regular menstrual cycles. Menarche and menstrual cycle regulation resemble mirrors that reflect adolescents’ future health (86). Therefore, moderating the disruptive factors in the developmental trajectory of the menstrual cycle can promote community health. More comprehensive basic and clinical studies are highly needed to show the pathways involved in the evolution of this trajectory.