1. Background
Since the beginning of the COVID-19 pandemic, some clinical manifestations have been reported for the respiratory system and other organ involvement. Angiotensin-converting Enzyme 2 (ACE2) receptors are detected in different organs such as cardiovascular, gastrointestinal, and endocrine systems (1). In the endocrine system, the intensity of these receptors is the highest in the testis, followed by the thyroid, and the least in the hypothalamus (2, 3). These receptors in the thyroid make the thyroid glands a potential target for virus entry (4).
In addition, there are other mechanisms for thyroid function test (TFT) abnormalities in COVID-19. Nonthyroidal illness is a well-known entity as the cause of thyroid test abnormalities in patients with a critical illness (5). During critical illness, the T3 level decreases rapidly mainly because of the decreased activity of deiodinase type-1 (D1), declined thyroid hormones’ binding to thyroid-binding globulin and other binding proteins, and decreased TSH secretion in prolonged critical illness (5, 6). Besides, COVID-19-related subacute thyroiditis is another reason for thyroid abnormality during or after the disease course. Clinical manifestations usually occur 2 - 6 weeks after COVID-19 infection, and patients show the typical manifestations of subacute thyroiditis, especially pain, in the thyroid region (7). Moreover, drugs such as corticosteroids and heparin, usually used for treating COVID-19 patients, can interfere with TFT, especially free T4 in the case of heparin use (8).
Some studies have been designed to evaluate TFT in COVID-19. Changes in TFT due to nonthyroidal illness and subclinical or overt thyrotoxicosis have been reported in COVID-19 (9, 10). However, data about overt thyrotoxicosis in COVID-19 are inconsistent (11, 12). Furthermore, some of these studies have assessed free T4 only in a subset of patients with low TSH (9, 10). The interference of heparin with the free T4 assay is another shortcoming in the studies of COVID-19 patients. In these circumstances, indirect methods are recommended for total T4 and TBG measurement and Free Thyroid Index (FT4I) assessment (8, 13).
2. Objectives
This prospective observational study was designed to examine the different features of TFT abnormalities, including free T4 abnormalities, in COVID-19 using direct and indirect methods of free T4 assessment.
3. Methods
This prospective cross-sectional study was conducted on 131 adult hospitalized COVID-19 patients at Booali-Sina Hospital, Qazvin province, Iran, from May to June 2021. The COVID-19 diagnosis was confirmed by detecting the virus with the Polymerase Chain Reaction (PCR). Patients with known thyroid disease and those receiving contrast media or amiodarone during the last six months before hospitalization or dopamine during hospitalization were excluded from the study. Demographic characteristics, symptoms, and underlying diseases were recorded in questionnaires.
3.1. Measures
Blood samples and ECG were obtained on the third day of hospitalization. Serum TSH, T3, total T4, free T4, and T3-uptake (TBI) were measured by electrochemiluminescence (ECL) method on the Roche/Hitachi cobas® 6000 immunoassay system using Roche kits. The free T4 index (FT4I) was calculated as T4/TBI. The TSH reference range was 0.27 - 4.2 µU/mL with the minimum detection limit of 0.005 µU/mL. The reference ranges of T3, T4, free T4, and FT4I were 0.8 - 2 ng/mL, 4.8 - 12.7 µg/dL, 0.93 - 1.7 ng/dL, and 4.8 - 12.7 µg/dL, respectively.
Subclinical/overt thyrotoxicosis was defined as low TSH plus normal/high free T4 and normal/high T3. Nonthyroidal Illness (NTI) was defined as low T3, low/normal TSH, and low/normal free T4. Subclinical/overt hypothyroidism was defined as high TSH plus normal/low free T4. Isolated hyperthyroxinemia was defined as high free T4 or high FT4I in the presence of normal TSH and normal T3.
3.2. Statistical Analysis
The data were analyzed using SPSS-24. The Kolmogorov–Smirnov test examined the distribution of quantitative data. Logarithmic transformation was performed when the distribution was not normal. The t-test and Analysis of Variance (ANOVA) were run for comparing the transformed data. A chi-square test was used to compare the categorized data. Finally, P < 0.05 was considered significant.
4. Results
Of 131 patients entering the study, 15 were excluded because of a history of hypothyroidism in 14 patients and recent exposure to contrast media in one patient. The data of 116 patients were evaluated. The mean age of the participants was 61.0 ± 15.2 years, and 37.9% of them were female. The most common comorbidity was diabetes, with a frequency of 25.0%. Heparin and corticosteroids were used by 92.2 and 84.5% of the patients, respectively (Table 1).
| Variables | No. (%) |
|---|---|
| Total | 116 |
| Age (y); mean ± SD | 61.0 ± 15.2 |
| Sex (female) | 44 (37.9) |
| Complains | |
| Constitutional | 92(79.3) |
| Respiratory | 105 (90.5) |
| Gastrointestinal | 30 (25.9) |
| Musculoskeletal | 66 (56.4) |
| O2 sat. (%) | |
| > 93 | 6 (5.2) |
| 90 - 93 | 25(21.5) |
| < 90 | 85(73.3) |
| Comorbidity | |
| HTN | 26 (22.5) |
| IHD | 13 (11.2) |
| DM | 29 (25.0) |
| COPD | 5 (4.3) |
| Drugs a | |
| Heparin | 107 (92.2) |
| Corticosteroids | 98 (84.5) |
Abbreviations: O2 sat., capillary oxygen saturation without oxygen supply; HTN, hypertension; IHD, ischemic heart disease; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease.
a Drugs with potential interference with thyroid function test.
Table 2 represents the frequencies of thyroid hormone abnormalities. Low T3 was the most frequent abnormality (56.9%), followed by low TSH (18.1%). The frequencies of high FT4I and free T4 were 16.4 and 11.2%, respectively. Of patients, 6.9% had high TSH, and 2.6% had low free T4.
| Variables | No. (%) |
|---|---|
| TSH | |
| Low | 21 (18.1) |
| High | 8 (6.9) |
| T3 | |
| Low | 66 (56.9) |
| High | 0 |
| T4 | |
| Low | 0 |
| High | 7 (6) |
| Free T4 | |
| Low | 2 (2.6) |
| High | 13 (11.2) |
| FT4I | |
| Low | 0 |
| High | 19 (16.4) |
Abbreviations: TSH, thyroid stimulating hormone; T3, triiodothyronine; T4, thyroxine; FT4I, free T4 index.
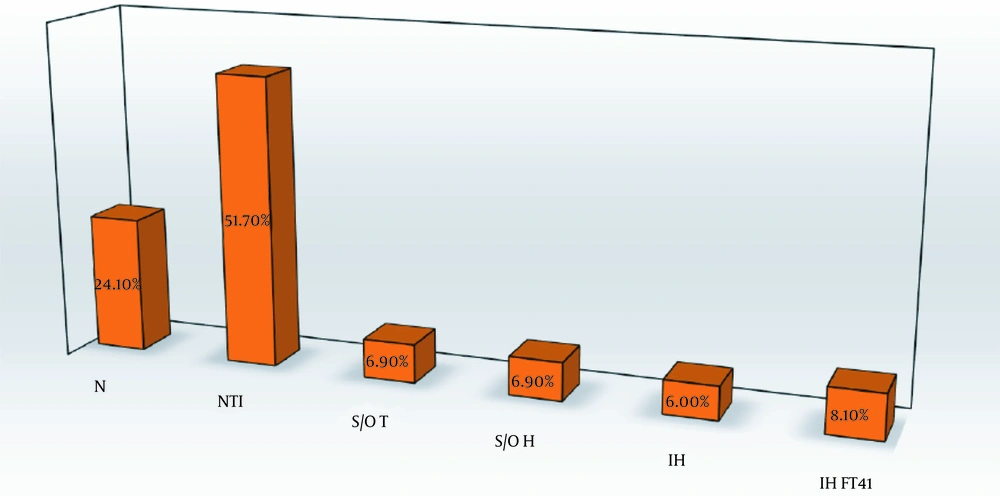
The frequencies of different TFT abnormalities are shown in Figure 1. Nonthyroidal illness had the highest frequency at 51.7% (60 patients). The frequency of both subclinical/overt hypothyroidism and subclinical/overt thyrotoxicosis was 6.9% (eight patients). Overt thyrotoxicosis was found in one patient in the subclinical/overt thyrotoxicosis group. Isolated hyperthyroxinemia and isolated high FT4I (high free T4 or high FT4I in the presence of normal TSH and T3) were found in 6% (seven patients) and 8.1% (nine patients), respectively. Among the participants, 28 had normal TFT. Five patients did not fulfill the criteria of any TFT groups; therefore, they were not categorized.
Differences in clinical presentation, paraclinical findings, and outcomes of different thyroid function groups are presented in Table 3. The sex distribution was significantly different among the groups. Notably, 75.0% of overt/subclinical hypothyroid patients were female. On the contrary, no patient in overt/subclinical thyrotoxicosis was female (P = 0.02). There was no significant difference in the chief complaints among the TFT groups. Among paraclinical findings, the lymphocyte percent was significantly lower in overt/subclinical thyrotoxicosis than in the other four groups (P = 0.002). Furthermore, the lymphocyte percent was lower in the NTI group than in the normal group (14.4 ± 7.5% vs. 21.2 ± 16.7%, P = 0.031).
| Variables | Normal TFT; N = 28 | NTI; N = 60 | Subclinical/ Overt Hypothyroidism; N = 8 | Subclinical/ Overt Thyrotoxicosis; N = 8 | Isolated Hyperthyroxinemia; N = 7 | P-Value |
|---|---|---|---|---|---|---|
| Age | 55.1 ± 14.4 | 62.7 ± 15.0 | 53.9 ± 16.5 | 64.8 ± 15.1 | 62.3 ± 12.3 | 0.09 |
| Sex (females) | 8 (28.6) | 26 (43.3) | 6 (75.0) | 0 | 2 (28.6) | 0.020 |
| Complaints | ||||||
| Constitutional | 22 (78.6) | 47 (78.3) | 8 (100) | 6 (75.0) | 6 (85.7) | 0.659 |
| Respiratory | 25 (89.3) | 55 (91.7) | 7 (87.5) | 7 (87.5) | 6 (85.7) | 0.977 |
| Gastrointestinal | 8 (28.6) | 16 (26.7) | 2 (25.0) | 1 (12.5) | 3 (42.9) | 0.772 |
| Musculoskeletal | 16 (57.6) | 34 (56.7) | 5 (62.5) | 4 (50.0) | 6 (85.7) | 0.639 |
| Paraclinical findings | ||||||
| CRP | 42.4 ± 39.4 | 79.0 ± 73.9 | 111.4 ± 97.0 | 96.6 ± 104.6 | 64.5 ± 67.3 | 0.191 |
| WBC | 8500.0 ± 3664.8 | 8649.1 ± 4242.1 | 5587.5 ± 1940.9 | 9462.5 ± 3022.3 | 8514.2 ± 2576.4 | 0.147 |
| Lymphocytes (%) | 21.2 ± 16.7 | 14.4 ± 7.5 | 18.4 ± 8.9 | 7.3 ± 3.5 | 16.5 ± 12.7 | 0.002 d |
| PMN (%) | 75.1 ± 12.1 | 79.7 ± 9.1 | 77.9 ± 9.2 | 84.1 ± 7.2 | 75.1 ± 14.9 | 0.136 |
| Atrial fibrillation | 0 | 1 (1.7) | 0 | 3 (37.5) | 0 | < 0.0001 e |
| Interfering drugs | ||||||
| Corticosteroids | 25 (89.3) | 50 (83.3) | 6 (75) | 6 (75.0) | 7 (100) | 0.564 |
| Heparin | 26 (92.9) | 54 (90.0) | 8 (100) | 8 (100) | 6 (85.7) | 0.719 |
| Outcomes | ||||||
| ICU admission | 3 (10.7) | 16 (26.7) | 1 (12.5) | 2 (25.0) | 1 (14.3) | 0.464 |
| Mortality | 1 (3.6) | 9 (15) | 1 (12.5) | 1 (12.5) | 0 | 0.476 |
a Values are expressed as No. (%) or mean ± SD.
b Five patients did not fulfill the characteristics of any groups of thyroid function test.
c Logarithmic transformation was used to compare non-parametric quantitative data.
d Significant differences between overt/subclinical thyrotoxicosis and other four groups (vs. normal group: P < 0.001, vs. overt/subclinical hypothyroidism: P = 0.002, vs. NTI: P = 0.004, vs. isolated hyperthyroxinemia: P = 0.028) and between NTI and normal group, P = 0.031.
e Significant difference between overt/subclinical thyrotoxicosis and other four groups.
Regarding atrial fibrillation (AF), in total, four patients were found to have this arrhythmia, and three (75%) were in the overt/thyrotoxic group. The fourth patient with AF was in the NTI group. The frequency of AF was 37.5% in overt/subclinical thyrotoxicosis versus 1.7% in NTI and nil in the other three groups (P < 0.001).
Two outcomes of ICU admission and expire rate were not significantly different among the groups.
5. Discussion
A wide spectrum of TFT abnormalities from NTI to isolated hypothyroxinemia was found in this study. About half of the patients had NTI. The other three abnormalities of subclinical/overt thyrotoxicosis, subclinical/overt hypothyroidism, and isolated hyperthyroxinemia had nearly similar frequencies (about 7%) in our patients.
Some TFT abnormalities such as NTI are widespread in different diseases, especially among critically ill patients. However, some other features, such as thyrotoxicosis, occur more specifically in COVID-19 patients. In the study by Muller, about 15 and 2% of COVID-19 patients in high and low-intensity care units (HICU and LICU) had thyrotoxicosis, respectively (9). In the studies by Lania et al. and Lui et al., thyrotoxicosis was found in 20.2 and 5.2% of COVID-19 patients, respectively (10, 14).
The exact pathophysiology of overt or subclinical thyrotoxicosis in COVID-19 is not clear. Subacute thyroiditis is one of the reported complications of COVID-19. These patients have typical features, including pain in the thyroid (7, 15, 16). No patient with overt/subclinical thyrotoxicosis in the studies mentioned above, and our study complained of neck pain. Therefore, typical subacute thyroiditis cannot justify this finding.
The thyroid is an organ with a high expression of ACE2 receptors. Thus, the COVID-19 virus can potentially enter thyroid cells and theoretically stimulate thyroid cells directly (3). However, no coronavirus was found in thyroid tissues based on death autopsy reports of expired COVID-19 patients (17). Unfortunately, these studies are minimal, and no patients might have thyrotoxicosis during COVID-19 before death.
The effects of cytokines on the thyroid are the other possible cause of atypical thyroiditis and releasing thyroid hormones from the thyroid (18). In the Lania et al. study, thyrotoxic groups had higher inflammatory markers than non-thyrotoxic groups (10). In our study, the CRP level in overt/subclinical thyrotoxicosis was similar to other groups, and only the percent of lymphocytes was significantly lower in the thyrotoxic group.
Differences in clinical features with typical subacute thyroiditis, especially pain, can be due to a lack of giant cell formation because of lymphopenia. Wei et al.’s study of thyroid pathology in patients with severe acute respiratory syndrome (SARS) found distortion and collapse of follicular architecture. Researchers have concluded that apoptosis may play a role in thyroid abnormalities' pathogenesis in SARS (19).
Hypothalamic-pituitary-thyroid axis involvement is one of the potential mechanisms for justifying the high rate of TSH suppression in COVID-19. The ACE2 receptors are present in the hypothalamus, and theoretically, the virus can affect TSH secretion (20). Patients with central hypothyroidism and hypocortisolism after the cure of SARS-COV-1 have been reported previously (21). In addition, corticosteroids can suppress TSH secretion (22). However, in these two situations, we expect low free T4 levels. In our study, overt/subclinical thyrotoxicosis was defined as high/normal free T4 in the presence of suppressed TSH. Therefore, central hypothyroidism or corticosteroid use cannot justify the TFT abnormality in this group of our patients.
One of the less reported features of TFT abnormality in our study was isolated hyperthyroxinemia. About 6 and 7% of our study patients had isolated high free T4 and isolated high FT4I, respectively. Some studies like Lania et al. and Muller et al. measured the TSH level in all patients, but the free T4 level was assessed only in a subset of patients with low TSH (9, 10). Therefore, isolated hyperthyroxinemia has not been reported in these studies. There are several pathophysiological mechanisms for justifying the findings of isolated hyperthyroxinemia.
Heparin activation lipoprotein lipase in vivo increases the levels of non-esterified fatty acid (NEFA) in vitro (23). High NEFA levels compete with T4 in binding to thyroid-binding proteins (24). In these situations, evaluating total T4 or using indirect methods is recommended for free T4 assessment, such as measuring TBG and calculating FT4I (8). Heparin was used by more than 90% of our patients; however, we found isolated hyperthyroxinemia with other T4 measures such as total T4 and FTI.
The second potential mechanism for isolated hyperthyroxinemia is related to the early stages of NTI. Michalaki et al.’s study (25) evaluated TFT changes during the first hours of surgery. The serum levels of total and free T4 were increased soon after the skin incision and remained high on the first day after the surgery. We assessed TFT on the third day of hospitalization, so this hypothesis was less plausible for our patients.
The third possible mechanism of isolated hyperthyroxinemia was the rapid conversion of the euthyroid state into thyrotoxicosis and the lack of enough time for TSH suppression. We could not exclude or approve this possibility in our study.
One of the most exciting findings of our study was the strong association of AF with overt/subclinical thyrotoxicosis (37.5% in this group vs. 1.5% in the NTI group and 0% in the other three groups, P < 0.001). Atrial fibrillation was found in four of our study patients, including three in the overt/subclinical thyrotoxicosis group. The fourth patient had nearly suppressed TSH (TSH = 0.3 mu/mL), normal free T4, and low T3; thus, we categorized this patient in the NTI group. In the study by Lania et al., 32.3% of the patients with COVID-19 and thyrotoxicosis had AF (10).
Based on a survey of electrophysiology professionals, atrial fibrillation is the most common arrhythmia in COVID-19 patients (26). New-onset AF has been reported in 3.6 - 6.7% of COVID-19 patients (27). Several mechanisms such as myocardial injury by the virus, hypoxemia, and sympathetic overactivity have been raised as the possible causes of AF susceptibility in COVID-19 patients (28). However, based on our work and Lania et al.'s studies, overt or subclinical thyrotoxicosis may have an essential role in this prevalent arrhythmia among COVID-19 patients (10).
The main limitation of our study was its cross-sectional design. Therefore, the natural course of TFT changes remained unknown.
In conclusion, our study revealed a wide spectrum of TFT changes in hospitalized COVID-19 patients. Some features, such as isolated hyperthyroxinemia, were less known. The strong association of atrial fibrillation with overt or subclinical thyrotoxicosis in our study necessitates other studies in this field and may provide a clue for the better management of AF in hospitalized COVID-19 patients.