1. Background
The prevalence of type 2 diabetes is rising worldwide (1). Type 2 diabetes accounts for about 90–95% of diabetes cases (2). In type 2 diabetes, hyperglycemia usually coexists with other cardiovascular risk factors and this makes the cardiovascular disease the most common cause of death among adults with diabetes mellitus. This further emphasizes the importance of the risk factor management in diabetic patients (3).
Nutrition therapy and a healthful eating pattern play an important role in managing diabetes and reducing the potential complications related to poor glycemic, lipid, and blood pressure control (4). Reduction in energy intake in overweight or obese adults has been emphasized in nutrition therapy for type 2 diabetes (5). In addition, one of the most commonly used dietary modifications consists of decreasing carbohydrate content of the diet; yet the role of this modification in cardiovascular risk factors is controversial (6). Lower carbohydrate diets (< 40% of kcal as carbohydrate) have been reported to improve markers of glycemic control and insulin sensitivity in patients with type 2 diabetes (4). Furthermore, improving carbohydrate quality through increasing dietary fiber intake may modify effects of higher carbohydrate diets on glycemic control (5, 6). Increasing fiber intake through diet may require substantial dietary changes. However, evidence indicates that adding fiber supplements in moderate amounts to a daily diet of patients with diabetes may lead to some improvements in glycemia and cardiovascular risk markers (7). In particular, soluble fiber has been related to lower levels of insulin resistance in non-diabetic subjects (8) and supplemental fiber from psyllium has been reported to have beneficial effects in type 2 diabetes (9).
Despite the benefits of nutrition therapy and diet program, for many individuals with diabetes, the most challenging part of the treatment plan is determining what to eat (10) and some patients may not have willingness or the potential to follow a structured diet program and may prefer simple dietary advice, such as those aimed at modifying dietary carbohydrate, including avoidance of added simple sugars and limiting consumption of refined carbohydrates.
We aimed to examine in patients with type 2 diabetes whether 1) two low-calorie diet programs based on diabetic exchange list are more effective than a simple dietary advice aimed at modifying carbohydrate intake to improve glycemic control and, 2) between the two low-calorie diet programs, a diet containing moderate amount of carbohydrate if supplemented with fiber powder (psyllium) will have a better impact than a lower carbohydrate, lower fiber diet on glycemic control. Finally, given that inflammation is considered to be an important mediator of cardiometabolic dysfunctions seen in type 2 diabetes (11), we explored the effect of this intervention on interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α), two well-known inflammatory cytokines (12).
2. Methods
2.1. Participants and Study Design
This pilot clinical trial was a randomized single-blind, parallel group design with three different arms, performed in compliance with the Helsinki declaration. Participants had an equal probability of assignment to the groups. The assignment to the groups was performed by using sealed envelopes and nutritional and biochemical analysis was performed in a blinded manner. The intervention lasted for 8 weeks. Ethical approval for the trial was obtained from the Ethics Committee of National Nutrition and Food Technology Research Institute, Tehran, Iran. Written informed consent was obtained from all patients.
Potential participants with type 2 diabetes were recruited through advertisement in a regional health center, and by calling previous study participants from our research center who had expressed interest in further studies. Those interested attended a visit at the university outpatient Nutrition Clinic (Shahid Beheshti University of Medical Sciences). We enrolled 60 overweight or obese men and women who had diabetes duration of > 1 year, currently treated with oral diabetes medication, aged 30 - 70 years with a BMI > 25 kg/m2. In the case of female individuals, those not pregnant nor taking estrogen, or progesterone were enrolled. None of the patients was treated with insulin, none had impaired renal, or liver function.
At baseline, demographic, anthropometric, physical activity and medical history were obtained. Physical activity level was assessed based on the frequency and time spent on common daily activities expressed as metabolic equivalent hours. Waist circumference was measured midway between the lowest rib margin and the iliac crest, with flexible, non-stretch fiberglass tape. Resting blood pressure was measured on two occasions using a sphygmomanometer (Riester, Jungingen, Germany) in the non-dominant arm by the same observer with five minutes between measurements and the mean value was taken. Venous blood samples were obtained in tubes containing sodium citrate as anticoagulant from patients who had fasted for at least 12 hours for the measurement of blood lipids, inflammatory markers, and glycemic control. Patients ingested their last oral therapy 12 hours before the test day. Fasting blood sampling and anthropometric assessments were repeated at the end of week 8.
2.2. Diet
After the baseline assessment, the participants were randomly assigned to one of the three parallel groups (n = 20 for each): simple nutritional advice (advice group), a calorie-restricted moderate carbohydrate diet plus psyllium (calorie-restricted, higher fiber: CRHF) or a calorie-restricted lower carbohydrate diet plus placebo (calorie-restricted, lower carbohydrate: CRLC). In the advice group, patients were given simple and generic dietary advice that is usually provided by healthcare professionals. The dietary advice given on this single occasion aimed to modulate carbohydrate intake: to reduce consumption of simple sugars and foods containing added simple sugars, avoidance of overeating too much refined carbohydrate such as white rice and white bread in each meal and replacement with whole grain foods such as whole wheat bread including traditional bread with added bran or industrial breads made from whole wheat. The two calorie-restricted diet programs were developed for each patient based on anthropometric and physical activity assessments. During the week 0 visit, daily estimated resting energy expenditure (REE) for each participant was computed using the Mifflin St. Jeor equation and the predicted REE was multiplied by the level of physical activity (estimated based on daily activities) to calculate total energy requirement (13). The participants were prescribed energy intake deficits of 25% of the total calculated energy requirements. Daily energy levels prescribed ranged between 1,100 kcal and 1,800 kcal. The calorie was distributed as 40% carbohydrate, 20% protein, and 40% fat in the CRLC and as 55% carbohydrate, 20% protein, and 25% fat in the CRHF group. In the CRLC, this would provide the minimum daily carbohydrate recommendation (130 g) in a typical calorie-reduced diet containing 1,300 kcal/d (14).
The programs were created based on the standard diabetic food exchange list for meal planning and participants were provided with a dietary instruction sheet and several sample menus. In addition, in the two calorie-restricted groups, patients were instructed to ingest a tablespoon of psyllium powder (approximately 7 g, containing by weight 3.1 g psyllium husk a product from Irandarouk company) in the CRHF or a tablespoon of placebo powder (containing corn starch) in the CRLC group, dissolved in one glass of water in the evening.
Four 24-hour recalls, two at baseline (one by face-to-face interview and one by telephone) and two at the week 8, were completed with each study subject. Recall data were analyzed using the Nutritionist software (version IV, N-Squared Computing, San Bruno, CA, USA). In the two low-calorie diet groups, dietary compliance was assessed by 24-h recalls obtained at the end of the intervention (week 8). Estimated carbohydrate, fat, and protein intakes from recalls were averaged and divided by the prescribed amounts of these nutrients according to the calorie requirements of each patient. The participants of the three groups were asked not to change their habitual physical activity levels for the duration of the study.
2.3. Biochemistry Analysis
Fasting blood samples were spun immediately following collection and plasma was separated, aliquoted, and frozen at -80°C for later analysis. Glucose and lipid profiles, as well as lipoprotein (a) (LPa), were analyzed in batches with autoanalyzer Selectra 2 (Vital Scientific, Spankeren, The Netherlands), using commercial kits (Pars Azmoon, Karaj, Iran). Commercially available ELISA kits were used to measure insulin (Diametra, Perugia, Italy), IL-6, and TNF-α (eBioscience, San Diego, USA). The assay sensitivity for insulin, TNF-α, and IL-6 were 0.25 µIU/mL, 4 pg/mL, and 2 pg/mL, respectively. Insulin resistance was calculated using the homeostasis model assessment of insulin resistance (HOMA-IR) score using standardized equations (15).
2.4. Statistical Analysis
The sample size was calculated based on the following assumptions: α = 0.05 level, and 80% power to detect the difference between groups in the change in plasma TG of 45 mg/dL or more, which was considered a clinically relevant difference, and 30% attrition rate. A minimum of 20 subjects per treatment group was required.
Continuous data were expressed as mean ± SD for normally distributed parameters or the median±interquartile range (IQR) in the case of skewed data distribution. The Kolmogorov–Smirnov test was used to determine the normality of the distribution. The changes during the study period (i.e. week 8 value minus baseline value) are presented as change from the baseline variable. Differences between groups were analyzed using a one-way ANOVA model (for normally distributed parameters) or Kruskal-Wallis H (for skewed data distribution). When a significant difference was found between groups, a Tukey post hoc test or Mann-Whitney test was performed to determine the differences between group means. Mean changes within each group from baseline to the end of the trial were analyzed using a Student’s paired t-test or its nonparametric counterpart (Wilcoxon signed rank test). All analyses were performed using SPSS version 16.0 (IBM, Armonk, NY) statistical software. All tests were two-sided, and the level of significance was set at α = 0.05.
3. Results
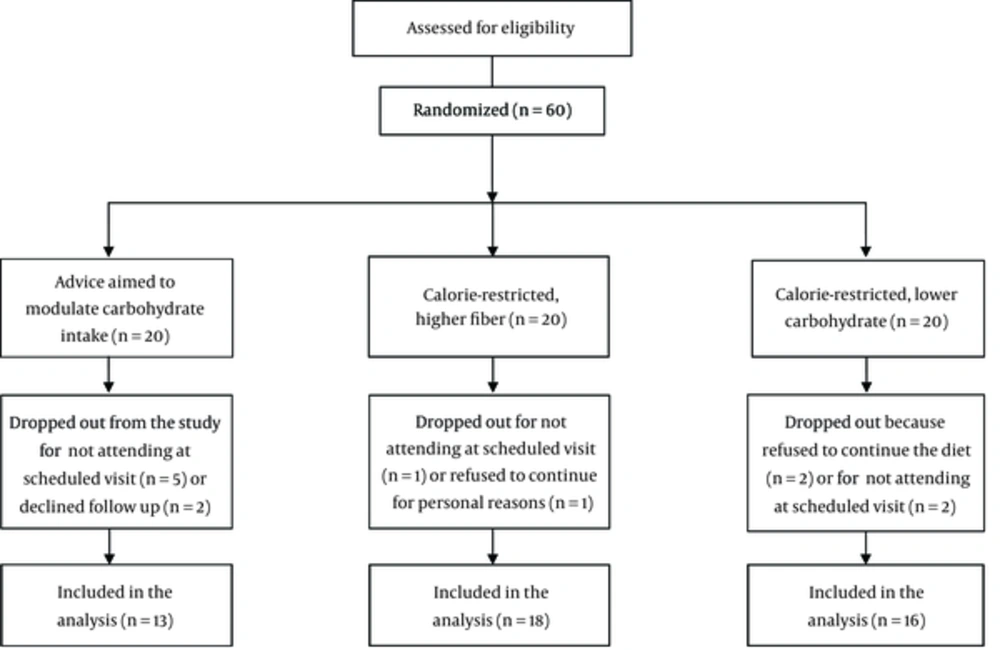
Of 60 patients enrolled in the study, 47 patients completed 8 weeks of the study period (Figure 1). Baseline characteristics of the participants are summarized in Table 1. There were no significant differences between study groups in terms of age, blood pressure, and the use of medications.
| Variable | Simple Advice (n = 13) | CRHF (n = 18) | CRLC (n = 16) | P Value |
|---|---|---|---|---|
| Age, y | 48.8 ± 8.5 | 55.9 ± 8.3 | 54.2 ± 7.2 | 0.10 |
| Sex (number of men) | 5 | 5 | 6 | 0.39 |
| Duration of diabetes, y | 6.5 ± 4.7 | 8.4 ± 7.3 | 7.4 ± 7.1 | 0.12 |
| BMI, kg/m2 | 29.7 ± 7.5 | 28.5 ± 2.5 | 30.3 ± 4.8 | 0.53 |
| Physical activity, Met-h/day | 20.4 ± 5.6 | 20.9 ± 5.0 | 21.4 ± 4.6 | 0.8 |
| Blood pressure Systolic, mmHg | 123.4 ± 10.8 | 126.4 ± 10.7 | 127.3 ± 21.6 | 0.8 |
| Diastolic, mmHg | 68.7 ± 13.6 | 71.0 ± 12.2 | 72.8 ± 10.3 | 0.5 |
| Medications | ||||
| Insulin release stimulator, % | 30 | 50 | 59 | 0.19 |
| Metformin, % | 90 | 95 | 95 | 0.86 |
| Statins, % | 30 | 55 | 45 | 0.20 |
| Aspirin, % | 15 | 35 | 25 | 0.32 |
| ACE-I/ARB, % | 20 | 30 | 47 | 0.31 |
| Plasma Creatinine, mg/dL | 0.9 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.2 | 0.56 |
Abbreviations: ACE-I, Angiotensin-converting enzyme inhibitor; ARB, Angiotensin receptor blocker; BMI, body mass index; CRHF, Calorie-restricted higher fiber; CRLC, Calorie-restricted, lower carbohydrate.
aValues are expressed as mean ± SD or percentages for dichotomous measures.
bNominal variables were compared using Chi-square Test.
3.1. Dietary and Anthropometric Data
Dietary information obtained from the 24-hour recalls is summarized in Table 2. The estimated current intake in CRHF and CRLC groups was 1476 ± 531 and 1735 ± 603, respectively, while their calculated total energy requirement for weight maintenance was 1713 ± 301 and 1864 ± 298 and their prescribed calorie was 1295 ± 216 and 1384 ± 203. After 8 weeks, energy intake significantly reduced in the CRHF group (P = 0.004). However, the difference between the groups was not statistically significant. Carbohydrate intake decreased significantly in all the three groups (advice, CRHF, and CRLC: P = 0.015, P = 0.001 and P = 0.005; respectively) with no significant difference between them. The amount of fat intake did not change. After 8 weeks, percent of calorie from protein increased in CRLC group but there was no significant difference between the groups. Soluble fiber intake increased significantly in the CRHF group (P < 0.001) with no change in other groups.
| Nutrient/Food | Advice | CRHF | CRLC | P Value |
|---|---|---|---|---|
| Energy, kcal/d | ||||
| Baseline | 1562.2 ± 411.3 | 1476.5 ± 531.7 | 1735.5 ± 603.0 | 0.3 |
| Week-8 | 1350.8 ± 366.4 | 1216.1 ± 446.1d | 1447.4 ± 377.2 | 0.2 |
| Carbohydrate, g/d | ||||
| Baseline | 220.7 ± 56.8 | 235.8 ± 95.1 | 263.5 ± 100.1 | 0.4 |
| Week-8 | 175.5 ± 50.3c | 175.3 ± 61.6d | 196.7 ± 49.2d | 0.4 |
| Carbohydrate, % | ||||
| Baseline | 57.5 ± 8.8 | 62.7 ± 8.5 | 60.7 ± 9.0 | 0.3 |
| Week-8 | 52.7 ± 9.5d | 57.9 ± 10.2 | 55.7 ± 10.2d | 0.4 |
| Protein, g/d | ||||
| Baseline | 67.2 ± 33.7 | 54.7 ± 17.3 | 60.5 ± 25.9 | 0.4 |
| Week-8 | 64.5 ± 27.392 | 52.2 ± 22.9 | 65.1 ± 25.0 | 0.2 |
| Protein, % | ||||
| Baseline | 15.5 ± 7.5 | 13.5 ± 4.8 | 11.6 ± 3.1 | 0.1 |
| Week-8 | 26.7 ± 24.1 | 15.9 ± 6.8 | 15.8 ± 5.2c | 0.057 |
| Fat, g | ||||
| Baseline | 47.1 ± 18.7 | 39.3 ± 19.3 | 53.2 ± 24.6 | 0.1 |
| Week-8 | 45.0 ± 23.1 | 37.0 ± 21.9 | 47.4 ± 22.8 | 0.3 |
| Fat, % | ||||
| Baseline | 27.0 ± 7.9 | 23.9 ± 7.4 | 27.6 ± 9.3 | 0.3 |
| Week-8 | 28.9 ± 9.5 | 26.1 ± 9.0 | 28.4 ± 8.2 | 0.6 |
| Cholesterol, mg/d | ||||
| Baseline | 161.8 ± 90.2 | 160.5 ± 146.8 | 225.3 ± 136.7 | 0.3 |
| Week-8 | 138.3 ± 90.2 | 142.0 ± 109.6 | 259.7 ± 188.4 | 0.28 |
| Soluble fiber, g | ||||
| Baseline | 3.7 ± 3.4 | 4.2 ± 3.3 | 4.5 ± 4.0 | 0.7 |
| Week-8 | 3.3 ± 3.0 | 8.1 ± 2.5d,e,f | 2.6 ± 3.3 | 0.001 |
| Simple sugars (sucrose, glucose, honey) | ||||
| Baseline | 21.3 ± 33.2 | 18.1 ± 18.5 | 23.4 ± 21.3 | 0.4 |
| Week-8 | 7.5 ± 28.5c | 18.2 ± 23.3 | 18.0 ± 1.7 | 0.2 |
| Foods groups, serving/d | ||||
| Milk/yogurt | ||||
| Baseline | 1.3 ± 1.1 | 1.3 ± 1.4 | 1.7 ± 2.8 | 0.4 |
| Week-8 | 1.3 ± 1.2 | 0.9 ± 1.0c | 1.6 ± 1.9 | 0.2 |
| Vegetables | ||||
| Baseline | 2.1 ± 2.6 | 2.4± 2.0 | 3.2 ± 3.3 | 0.3 |
| Week-8 | 2.0 ± 1.8 | 3.3± 2.7 | 2.8 ± 2.1 | 0.2 |
| Fruits | ||||
| Baseline | 3.0 ± 2.7 | 3.1 ± 2.7 | 3.5 ± 2.5 | 0.3 |
| Week-8 | 2.8 ± 2.5 | 3.5 ± 2.9 | 2.9 ± 3.1 | 0.2 |
| Starch/bread | ||||
| Baseline | 8.5 ± 3.9 | 8.6 ± 5.1 | 9.8 ± 6.9 | 0.2 |
| Week-8 | 7.0 ± 3.5 | 4.9 ± 4.6 | 5.9 ± 3.5c | 0.5 |
| Meat and substitutes | ||||
| Baseline | 4.0 ± 3.6 | 3.1 ± 2.3 | 3.2 ±2.3 | 0.1 |
| Week-8 | 3.7 ± 3.6 | 3.7 ± 3.1 | 4.9 ± 3.9 | 0.1 |
| Fats | ||||
| Baseline | 4.8 ± 2.9 | 4.8 ± 2.0 | 5.9 ± 3.1 | 0.4 |
| Week-8 | 4.4 ± 3.1 | 4.4 ± 3.1 | 5.4 ± 2.8 | 0.2 |
Abbreviations: CRHF, Calorie-restricted, higher fiber; CRLC, Calorie-restricted, lower carbohydrate.
aValues are expressed as mean ± SD.
bData were compared using one-way ANOVA with a post hoc Tukey test.
cP < 0.05 for within-group differences: Student’s paired t-test comparing week 0 to week 8 values.
dP ≤ 0.01 for within-group differences: Student’s paired t-test comparing week 0 to week 8 values.
eP < 0.05, Significantly different from advice group based on Tukey post hoc test.
fP < 0.01, Significantly different from CRLC group based on a Tukey post hoc test.
Dietary compliance (defined as estimated intake at week 8 divided by prescribed amount) for energy, carbohydrate, fat, and protein was respectively 0.94, 0.99, 1.04, and 0.80 in the CRHF group, and 1.05, 1.42, 0.78, and 0.95 in the CRLC group. These results indicate that protein intake in the CRHF group was lower than the prescribed amount as well as in the CRLC group; carbohydrate intake was higher while fat intake was lower than the prescribed amount.
Weight non-significantly reduced by 0.5 kg in two calorie-restricted groups. However, no significant differences were observed between the groups (Table 3). There were no significant changes in BMI and waist circumference within or between the groups. Systolic blood pressure decreased significantly in the CRHF group (P = 0.04) but the difference between the three groups was not statistically significant.
| Factors | Baseline | Week-8 | Changeb |
|---|---|---|---|
| Weight, kg | |||
| Simple advice | 78.1 ± 21.3 | 78.4 ± 21.8 | 0.1 ± 1.2 |
| CRHF | 72.3 ± 10.3 | 71.8 ± 10.4 | -0.5 ± 1.8 |
| CRLC | 78.7 ± 12.8 | 78.1 ± 13.5 | -0.6 ± 1.4 |
| BMI, kg/m2 | |||
| Simple advice | 29.7 ± 7.5 | 29.7 ± 7.7 | 0.0 ± 0.5 |
| CRHF | 28.5 ± 2.5 | 28.3 ± 2.4 | -0.2 ± 0.7 |
| CRLC | 30.3 ± 4.8 | 30.0 ± 4.9 | -0.3 ± 0.5 |
| Waist Circumference, cm | |||
| Simple advice | 96.5 ± 19.3 | 97.0 ± 19.3c | 0.5 ± 0.7 |
| CRHF | 97.9 ± 7.5 | 97.5 ± 7.6 | -0.4 ± 2.2 |
| CRLC | 103.3 ± 10.6 | 102.7 ± 10.6 | -0.5 ± 1.0 |
| Systolic BP, mmHg | |||
| Simple advice | 123.4 ± 10.8 | 129.2 ± 18.7 | 5.8 ± 19.3 |
| CRHF | 126.4 ± 10.7 | 122.3 ± 11.5c | -4.15 ± 8.6 |
| CRLC | 127.3 ± 21.6 | 126.2 ± 16.6 | -1.1 ± 10.0 |
| Diastolic BP, mmHg | |||
| Simple advice | 68.7 ± 13.6 | 74.4 ± 11.8 | 5.7 ± 15.2 |
| CRHF | 71.0 ± 12.2 | 71.15 ± 9.3 | 0.1 ± 9.0 |
| CRLC | 72.8 ± 10.3 | 73.6 ± 6.9 | 0.7 ± 9.8 |
Abbreviations: CRHF, Calorie-restricted, higher fiber; CRLC, Calorie-restricted, lower carbohydrate.
aValues are expressed as mean ± SD.
bData were compared using one-way ANOVA with a post hoc Tukey test.
cP < 0.05 for within-group differences: Student’s paired t-test comparing week 0 and week 8 values.
3.2. Plasma Biochemical Measures
Fasting plasma glucose decreased significantly in the CRLC group and the absolute change of glucose was significantly lower than that in the advice or CRHF group (estimated power was 0.69 and 0.67, respectively, based on α = 0.05 and two-tailed t-test). Fasting insulin levels significantly decreased in the CRHF group, and the absolute change of insulin was significantly lower when compared to the two advice and CRHF groups (estimated powers were 0.97 and 0.34, respectively). In addition, HOMA-IR decreased significantly in the CRHF group when compared to the two other groups (estimated powers were > 0.9 for both tests) (Table 4).
| Factors | Baseline | Week-8 | Changeb |
|---|---|---|---|
| Fasting Glucose, mg/dL | |||
| Simple advice | 134.5 ± 66.7 | 136.6 ± 40.5 | 2.5 ± 16.2 |
| CRHF | 136.5 ± 40.5 | 127.0 ± 73.2 | 3.5 ± 29.7 |
| CRLC | 147.0 ± 61.0 | 132.0 ± 31.0c | -27.0 ± 40.5d,e |
| Fasting insulin, µIU/mL | |||
| Simple advice | 2.7 ± 6.0 | 5.1 ± 8.0c | 1.3 ± 1.9 |
| CRHF | 3.0 ± 4.0 | 1.8 ± 3.1f | -1.0 ± 1.2g |
| CRLC | 1.2 ± 1.6 | 2.8 ± 2.9 | 0.3 ± 3.1h |
| HOMA-IR | |||
| Simple advice | 0.9 ± 2.2 | 1.8 ± 2.0 | 0.5 ± 0.7 |
| CRHF | 1.0 ± 1.4 | 0.7 ± 1.0c | -0.3 ± 0.6g |
| CRLC | 0.5 ± 0.7 | 0.9 ± 1.0 | 0.2 ± 0.9e |
Abbreviations: CRHF, Calorie-restricted, higher fiber; CRLC, Calorie-restricted, lower carbohydrate.
aValues are expressed as median ± IQR.
bValues between intervention groups were compared by Kruskal-Wallis H test.
cP < 0.05 for within-group difference: Wilcoxon signed rank test comparing week 0 and week 8 values.
dP < 0.05 significantly different from simple advice group based on a Mann-Whitney test.
eP < 0.05, Significantly different from CRHF group based on Mann-Whitney test.
fP ≤ 0.01 for within-group difference: Wilcoxon signed rank test comparing week 0 and week 8 values.
gP≤ 0.01, Significantly different from simple advice group based on a Mann-Whitney test.
hP ≤ 0.01, Significantly different from CRHF group based on a Mann-Whitney test.
Plasma triglyceride, cholesterol, LDL-C, HDL-C, and Lp (a) had no significant changes between the groups (Table 5). Plasma IL-6 significantly increased in the advice group and decreased in the CRLC group. The absolute change of plasma IL-6 was different between the groups, with the lower values in the CRLC and CRHF groups compared to the advice group (estimated power was > 0.9 for both tests). No significant difference was observed between the two calorie-restricted groups in terms of plasma IL-6. Plasma TNF-α significantly increased in the advice group and had a trend toward lower values in the CRHF group (P = 0.09). The absolute change of TNF-α was different between the CRHF and advice group (estimated power was 0.79), with no difference between the two calorie-restricted groups.
| Factors | Baselinea | Week-8 | Change from Baseline |
|---|---|---|---|
| Triglyceride, mg/dL | |||
| Simple advice | 127.3 ± 70.5 | 123.7 ± 43.7 | -3.6 ± 61.4 |
| CRHF | 135.7 ± 59.4 | 142.8 ± 57.1 | 7.1 ± 38.1 |
| CRLC | 125.9 ± 54.7 | 123.4 ± 48.0 | -2.4 ± 32.8 |
| Total cholesterol, mg/dL | |||
| Simple advice | 147.9 ± 52.2 | 152.5 ± 46.9 | 4.6 ± 25.3 |
| CRHF | 158.1 ± 35.8 | 156.1 ± 33.2 | -2.0 ± 16.5 |
| CRLC | 149.4 ± 33.5 | 152.2 ± 37.1 | 2.8 ± 32.7 |
| LDL-c, mg/dL | |||
| Simple advice | 87.9 ± 28.5 | 92.3 ± 34.4 | 4.4 ± 10.1 |
| CRHF | 92.1 ± 22.3 | 92.0 ± 19.3 | -0.1 ± 13.1 |
| CRLC | 86.3 ± 22.9 | 87.6 ± 23.9 | 1.2 ± 15.0 |
| HDL-c, mg/dL | |||
| Simple advice | 43.5 ± 7.3 | 44.8 ± 9.5 | 2.3 ± 8.5 |
| CRHF | 45.9 ± 10.4 | 45.6 ± 8.1 | -0.2 ± 7.4 |
| CRLC | 45.5 ± 8.4 | 46.7 ± 8.9 | 1.1 ± 7.5 |
| Lp(a), mg/dL | |||
| Simple advice | 18.4 ± 16.8 | 19.7 ± 18.2 | 1.3 ± 2.5 |
| CRHF | 17.25 ± 5.8 | 17.6 ± 7.2 | 0.4 ± 3.3 |
| CRLC | 21.9.3 ± 15.7 | 23.8 ± 16.4b | 1.9 ± 2.2 |
| TNF-α, pg/mL | |||
| Simple advice | 46.9 ± 32.1 | 67.6 ± 38.8b | 20.6 ± 32.0 |
| CRHF | 64.2 ± 47.3 | 53.6 ± 37.3 | -10.5 ± 27.2c |
| CRLC | 40.1 ± 44.0 | 42.2 ± 38.3 | 2.0 ± 16.4 |
| IL-6, pg/mL | |||
| Simple advice | 14.2 ± 10.3 | 21.7 ± 14.6d | 7.5 ± 6.8 |
| CRHF | 12.2 ± 10.1 | 11.1 ± 9.4 | -1.2 ± 4.7c |
| CRLC | 18.4 ± 10.8 | 14.1 ± 9.1d | -4.2 ± 5.6e |
Abbreviations: CRHF, Calorie-restricted, higher fiber; CRLC, Calorie-restricted, lower carbohydrate.
aValues are expressed as mean ± SD.
bP < 0.05 for within-group differences: Student’s paired t-test comparing week 0 and week 8 values.
cP < 0.01 significantly different from simple advice group based on a Tukey post hoc test.
dP ≤ 0.01 for within-group differences: Student’s paired t-test comparing week 0 and week 8 values.
eP ≤ 0.001 significantly different from simple advice group based on a Tukey post hoc test.
4. Discussion
This study compared the effects of a simple dietary advice and aimed to modulate carbohydrate intake with two energy-restricted diets with different carbohydrate and fiber contents on anthropometric, biochemical, and inflammatory markers over an 8-wk intervention period for individuals with diabetes. The results obtained indicate that the use of calorie-restricted diets based on the food exchange system has some favorable effects on plasma glucose, insulin, and inflammatory cytokines compared to the simple dietary advice. In addition, compared to CRLC, the CRHF diet significantly reduced plasma insulin and HOMA-IR index.
With regard to the dietary intake and loss of weight, although no significant differences in energy and macronutrient intake and body weight were observed between the groups, the patients in the two calorie-restricted groups had slightly greater reductions in energy intake (kilocalories per day) with slightly greater weight loss than those in the advice group. One reason for the lack of significant weight loss in two calorie-restricted groups may be related to modest calorie deficit prescribed for the calorie-restricted groups. Participants, especially those in the CRHF group did not have high calorie intakes and their calorie intakes were already slightly lower than the required amount for weight maintenance based on their calculated total energy requirement at baseline. This combined with a modest calorie deficit prescribed for the calorie-restricted groups (25% caloric restriction from calculated energy requirements) might explain the lack of significant weight loss in the two calorie-restricted groups during the study period. Although participants had relatively lower calorie intake than the general population, this may not be uncommon in many patients with type 2 diabetes who have the disease for several years with the intention of preventing extra weight gain. Compared to baseline, carbohydrate intake reduced in all of the three groups, which reflects the effectiveness of interventions in reducing the consumption of carbohydrate sources. Such a result was expected since in the two calorie-restricted groups meal planning for the restricted calorie was based on the specified number of carbohydrate exchanges, and in the advice group, moderation in consumption of carbohydrate sources was advised. However, despite a different macronutrients prescription for the two calorie-restricted diets, the dietary assessment showed the desired prescription percentage of calories from carbohydrate did not reach in CRLF diet and comparison of the two calorie-restricted groups indicated that dietary compliance with the prescribed carbohydrate was better in the CRHF than in the CRLC group. This could be related to the difficulty in adhering to a low carbohydrate diet in those diabetic patients that habitually have high carbohydrate intake. Soluble fiber intake increased in the CRHF group, which could be attributed to psyllium supplementation. Although modest, these dietary changes could have contributed to the differences observed between the groups.
Fasting plasma glucose decreased in the CRLC group compared to the advice or CRHF groups. This may be related to the type of carbohydrates consumed or a relatively greater reduction in carbohydrate intake in the CRLC diet. Plasma insulin and HOMA-IR values significantly reduced in the CRHF diet, compared to the two other groups. Increased insulin secretion could contribute to hyperinsulinemia and insulin resistance, both of which have central roles in the metabolic disturbances associated with obesity and type 2 diabetes (16). The results suggest that the amount of insulin required for controlling glucose is reduced with a CRHF diet. Increased soluble fiber intake from psyllium could have been contributed to this finding. These results are in agreement, at least to some degrees, with a previous study showing that psyllium supplementation (3.5 g three times a day) for 6 months without any calorie restriction, improved fasting insulin and HOMA index in addition to fasting blood glucose and glycated hemoglobin (17). In contrast, Ziai et al. demonstrated that supplementation of diet with psyllium (5.1 g twice a day) for 8 weeks reduced fasting blood glucose and glycated hemoglobin in patients with type 2 diabetes, but had no significant effect on fasting serum insulin (18). Although it is not possible to validate the mechanism of action of reduced insulin level in this study, it is probable that soluble fibers such as psyllium may delay the delivery of glucose to the circulation through gel-forming properties and, as a result, decrease insulin secretion (19). Furthermore, the fiber fermentation in the intestine produces short-chain fatty acids that have been shown to be effective in enhancing peripheral insulin sensitivity (20).
Another finding of our study is that both low-calorie diets reduced inflammation, as quantified by a reduction in IL-6 or TNF-α. Calorie restriction is known to accompany a reduction in inflammatory markers, including in IL-6 (21). In a short-term study of obese diabetic men by Khoo and colleagues, 8 weeks of a calorie-restricted low-fat, high-protein, reduced-carbohydrate diet led to a significant decrease in plasma IL-6 levels (22). Comparisons between the two low-calorie diets showed no difference regarding the effect on inflammatory cytokines. Increased fiber intake may partly explain more favorable changes in the inflammatory profile in chronic diseases including type-2 diabetes mellitus (23). In particular, soluble fiber may be important in this respect, because it is converted to the short-chain fatty acids, which have several anti-inflammatory properties such as regulation of cytokine release (24). In addition, soluble fiber intake may also modulate the immune response through alterations of the microbial composition in the gut (25). There are few studies addressing the impact of supplementary psyllium on inflammatory factors, such as IL-6 or TNF-α. In a study evaluating the effect of two high fiber diets in lean and obese subjects, psyllium supplementation for 3 weeks resulted in some reductions in CRP levels among lean individuals while only modest changes were observed in obese people (26). However, another investigation on overweight or obese adults with no history of heart disease failed to demonstrate effects of psyllium supplementation on serum CRP or IL-6 levels (27).
Plasma lipids were not different within or between the groups. Participants in our study had essentially desirable initial plasma triglyceride, LDL-C, and HDL-C levels. This might be the most likely explanation for the lack of effects on lipid profile.
There were a number of limitations to this study. The sample sizes in each group were rather small and the power to detect subtle changes is therefore restricted, especially in the case of fasting blood sugar and TNF-α. Furthermore, the dietary intake data were based on a limited number of days of dietary recall. In addition, the study would have benefited if continued for longer durations to assess maintenance. Moreover, we assessed a limited number of parameters related to glycemic control and inflammation.
In conclusion, the low-calorie diets that are based on the diabetic food exchange list can improve inflammatory markers in individuals with type 2 diabetes. Subjects with type 2 diabetes that habitually have high carbohydrate intake are encouraged to consume a moderate carbohydrate diet and soluble fiber sources to improve plasma insulin and metabolic factors and psyllium could be a good selection of soluble fiber for diabetics. However, more studies are needed to confirm the efficacy of these dietary modifications in metabolic profile and inflammatory status in subjects with type 2 diabetes.