1. Context
Pregnancy is associated with several changes in maternal thyroid function (1, 2) during various stages of pregnancy, that may cause a significant increase in the prevalence of thyroid disease in pregnant women (2). Accordingly, thyroid disease is one of the most common endocrine disorders during pregnancy (3).
Hyperthyroidism is caused by the hypersecretion of thyroid hormones. Moreover, there is evidence demonstrating that hyperthyroidism is associated with the increased excitability or hyper-metabolism of circulation, digestive system, and nerve (4). Earlier studies have demonstrated that not only overt thyroid dysfunction but also sub-clinical dysfunction can associate with increased risks of adverse maternal and fetal outcomes (3, 5-9). Subclinical hyperthyroidism is defined by low serum thyroid-stimulating hormone (TSH), but normal serum T4 and T3 concentrations (10), which may reflect mild thyroid hormone excess, hypothalamic, or pituitary disease (11). This thyroid dysfunction is relatively common among women of reproductive age and increases with aging (12). Subclinical hyperthyroidism occurs in 0.4 - 1.7% of pregnancies (12-16) and it is more prevalent in parous women in iodine deficiency areas (17, 18).
Despite solid evidence regarding the adverse effect of maternal hyperthyroidism on pregnancy outcomes (2, 16, 19-22), the results of studies conducted on adverse consequences of subclinical hyperthyroidism on pregnancy outcomes are still conflicting and inconclusive (2, 3, 9, 12, 16, 22-25). While some studies reported no significant adverse effect of subclinical hyperthyroidism on pregnancy outcomes (5, 12, 26, 27), others reported protective effects in terms of pregnancy-induced hypertension (12), and improvement of Apgar score (25), and some other ones illustrated adverse effects on preeclampsia and placental abruption (2).
Several previous systematic reviews and meta-analyses have been published on adverse pregnancy outcomes of thyroid dysfunction so far (8, 25, 28-31); however the majority of them have not reported these outcomes specifically for subclinical hyperthyroidism (8, 28, 29). Among those ones that included this subgroup of thyroid dysfunction, various feto-maternal outcomes have not been investigated and only intrauterine growth restriction (IUGR) (25) and low birth weight (LBW) (30) have been reported.
2. Objectives
Accordingly, this systematic review and meta-analysis aimed to investigate the association between subclinical hyperthyroidism and various adverse pregnancy outcomes in pregnant women.
3. Evidence Acquisition
3.1. Study Design
The present study was approved by the ethics committee of the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences (code: IR.SBMU.ENDOCRINE.REC.1399.073), and the study was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (Code: CRD42020192583, link: www.crd.york.ac.uk/prospero/#myprospero). The systematic review and meta-analysis became carried out based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (32) and MOOSE guidelines (33).
The PICO question of this study was: in pregnant women with subclinical hyperthyroidism what are adverse pregnancy outcomes, compared to those in the euthyroid group?
3.2. Search Strategy and Study Selection
A comprehensive literature search was conducted using PubMed [including Medline] and Scopus and Web of Sciences databases for retrieving English articles published until January 2022 investigating adverse pregnancy outcomes in women with subclinical hyperthyroidism. Furthermore, to maximize the identify of eligible research, review articles and the reference lists of the included research had been manually evaluated as well.
The following keywords, alone or in combination, had been used for the search: ((“subclinical hyperthyroidism” OR “subclinical thyroid dysfunction “OR “subclinical thyroid disorder”) AND (“pregnancy” OR “gestational” OR “maternal” OR “pregnant women” ) AND (“adverse pregnancy outcomes” OR “pregnancy outcomes” OR “pregnancy complications” OR “maternal outcome” OR “perinatal outcome” OR “neonatal outcome” OR “infant outcome” OR “miscarriage” OR “pregnancy loss” OR “abortion” OR “stillbirth” OR “fetal death” OR “gestational diabetes” OR “gestational hypertension” OR “preeclampsia” OR “PIH” OR “postpartum hemorrhage” OR “hemorrhage” OR “PPH” OR “Placenta abruption” OR “placenta previa” OR “premature rupture of membrane” OR “PROM” OR “oligohydramnios” OR “preterm” OR “small for gestational age” OR “Intra uterine growth restriction” OR “IUGR” OR “Low birth weight” OR “LBW” OR “Apgar” OR “fetal distress” OR “neonatal distress” OR “RDS” OR “neonatal death” OR “neonatal mortality” OR “NICU admission” OR “neonatal admission” OR “anomalies” OR “malformation” OR “neonatal *” OR “maternal *” OR “fetal *”)) (Appendix 1).
3.3. Eligibility Criteria, Study Selection, and Data Extraction
In the present meta-analysis, we searched all observational studies (cohort, case-control, and cross-sectional) published in English. Studies have been eligible if they had: (1) A study population including pregnant women with subclinical hyperthyroidism, (2) the pregnant women had not received any treatment, (3) a control group of euthyroid, and (4) evaluated at least one adverse pregnancy outcome, including pregnancy loss (stillbirth, fetal death, abortion, or perinatal mortality), pregnancy-induced hypertension (PIH), preeclampsia or eclampsia, gestational diabetes, obstetrical hemorrhage (PPH, placenta abruption, and placenta previa), preterm delivery, premature rupture of membrane (PROM), preterm premature rupture of the membranes (PPROM), small for gestational age (SGA) or IUGR, low birth weight (LBW), large for gestational age (LGA), macrosomia, malpresentation, respiratory distress syndrome (RDS), fetal distress, low Apgar score, neonatal death, neonatal complication or NICU admission. We have also excluded non-original studies, including case reports, review articles, animal studies, guidelines, commentaries, editorials, meeting abstracts, and letters to the editor, as well as the researches that did not offer accurate and clear data in this regard. Additionally, studies on women with multiple pregnancies and those with no control group were excluded.
The screening of titles, abstracts, and full-text of the retrieved researches was performed independently by two independent authors (FRT and SN) to select eligible studies. The disagreements among the reviewers were resolved through scientific discussions. Information was extracted from each selected article, including study characteristics, such as the first author’s name, publication year, country of study, journal name, article title, sample size, study design, population characteristics, pregnancy outcomes, and test results. To prevent extraction and data entry errors, the control checking between the original publications and the final data used in the systematic review was performed by all authors.
3.4. Quality Assessment and Risk of Bias
The modified Newcastle-Ottawa Quality Assessment Scale (NOS) was used to assess the quality of methods and results of the selected studies (34). Two reviewers, who were blinded to the journal name, study’s author, and institution, separately evaluated the quality of each study. Information on selection, comparability, and outcomes was evaluated for selected studies. Studies with 3 or 4 stars in the selection domain, 1 or 2 stars in the comparability domain, and 2 or 3 stars in the outcome/exposure domain were considered good quality, studies with 2 stars in the selection domain, 1 or 2 stars in the comparability domain, and 2 or 3 stars in outcome/exposure domain were considered as the fair quality, and studies with 0 or 1 star in selection domain, 0 stars in comparability domain, or 0 or 1 stars in outcome/exposure domain were considered as poor quality.
We have also assessed the risk of bias for all included studies using the Cochrane Collaboration’s tool for cohort studies (35) that evaluates seven domains of risk of bias, including adequacy of follow up of cohorts, outcome assessment, the assessment of the presence or absence of prognostic factors, control of prognostic variables, presence of the outcome of interest at the start of the study, the assessment of exposure, and the selection of exposed and non-exposed cohorts. Review authors’ judgments were categorized as “definitely No (low risk of bias)”, “probably no”, “definitely yes (high risk of bias)”, and “probably yes” as low risk, high risk, and unclear risk of bias, respectively.
3.5. Outcome Measures
Maternal, neonatal and fetal outcomes of interest were categorized into seven composite outcomes, including (1) hypertensive disorders (PIH, preeclampsia and severe preeclampsia, eclampsia), (2) preterm delivery (preterm < 34 weeks, and < 37 weeks), (3) macrosomia/LGA (macrosomia, large for gestational age), (4) pregnancy loss (abortion, fetal death, fetal loss, still birth, perinatal mortality, medically induced labors), (5) adverse maternal outcomes (PIH, preeclampsia and severe preeclampsia, eclampsia, gestational diabetes, obstetrical hemorrhage (PPH, placenta abruption, and placenta previa)), (6) adverse fetal outcomes (preterm (< 37 weeks and < 34 weeks)), premature rupture of membrane (PROM), preterm premature rupture of the membranes (PPROM), IUGR/small for gestational age (SGA), low birth weight (LBW), very LBW and extremely LBW, macrosomia, LGA, malpresentation, fetal distress, (7) adverse neonatal outcomes (respiratory distress syndrome (RDS), low Apgar score, neonatal death, neonatal complication (hyperbilirubinemia, respiratory distress, sepsis, hypoglycemia, hypothermia, intracranial bleed, Umbilical artery blood pH < 7.0, intraventricular hemorrhage, necrotizing enterocolitis), NICU admission).
3.6. Statistical Analysis
The software package STATA (version 12; STATA Inc., College Station, TX, USA) was applied to perform statistical analyses. Heterogeneity was evaluated using the I-squared (I2) statistics (values above 50% were interpreted as significant heterogeneity) and P < 0.05 obtained from the chi-squared test was interpreted as heterogeneity. The funnel plot and Egger test were used for publication bias assessment; the trim and fill method was conducted in case of significant results to adjust the bias.
The random-effects model was employed for heterogeneous findings and for calculating the pooled odds ratio (OR). For all studies, raw data extracted (the number of events in the case and control group) and OR were estimated for them. The inverse variance weighting method was used to calculate the pooled OR.
Furthermore, sensitivity analyses are used for the robustness of the results, any single study with a heterogeneity value out of the range of 95% CI was considered influential and excluded.
P < 0.05 was set as the significance level.
4. Results
4.1. Study Selection and Study Characteristics
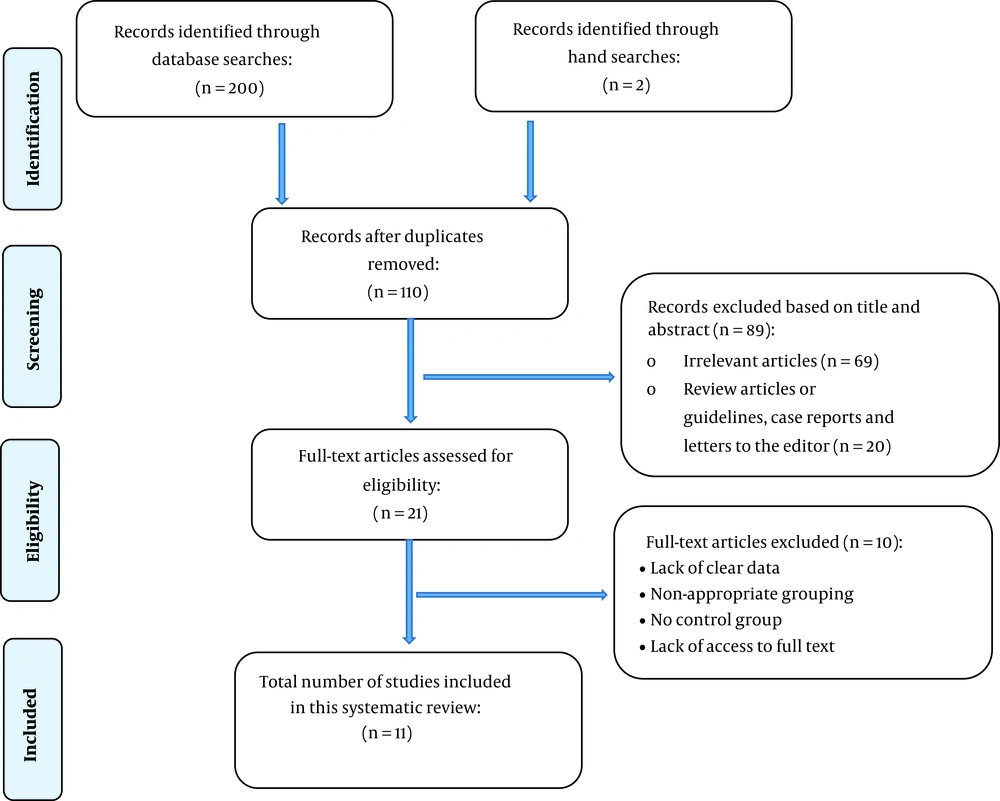
Of 202 records retrieved through searching databases, 11 studies were included, and all of them had a cohort design (2, 3, 9, 12, 22-24, 36-39). Table 1 summarizes the characteristics of the studies included.
| First Author (y) | Country | Study Design | Subclinical Hyperthyroidism Definition | Sample Size | Subclinical Hyperthyroidism/Euthyroid | Prevalence | Outcome | Adjusted Factors | Quality Assessment |
|---|---|---|---|---|---|---|---|---|---|
| Casey (12) | USA | Cohort | thyroid-stimulating hormone (TSH) < 5th percentile (0.2 mU/L) | 25,765 | 433/23124 | 433 (1.7%) | Pregnancy outcomes: Hypertensive disorders (gestational hypertension, severe preeclampsia), diabetes, placental abruption, weeks of gestation at delivery, cesarean delivery. neonatal Outcomes: Birth weight, intensive care nursery, Apgar score at 5 minutes ≤ 3, umbilical artery blood pH < 7.0, necrotizing enterocolitis, respiratory distress syndrome, major malformations, intraventricular hemorrhage, fetal death, neonatal death, perinatal mortality | Race and parity | Good |
| Mannisto (36) | Northern Finland | Prospective Population-Based Cohort | TSH < 5th per. (0.19 mU/L), fT4 5th - 95thper. (11.96 - 20.5 pmol/L) | 9247 | 204/4719 | 204 | Preterm delivery; birth measurements (birth weight, small for gestational age (SGA) and large for gestational age (LGA) infants, birth length, ponderal index (birth weight/birth length3), head circumference); Apgar scores; perinatal mortality (stillborn and early neonatal deaths (< 7 d after birth)); neonatal deaths; malformations; presentation at birth; mode of delivery; absolute and relative placental weight; and umbilical cord length. | Maternal age and parity | Good |
| Taghavi (39) | Iran | Cohort | TSH < 0.4 mU/L and normal FT4 level | 500 | 21/427 | 21 (4.2%) | Preeclampsia, preterm | - | Fair |
| Sahu (9) | India | Prospective cohort | TSH < 0.5 mIU/L | 633 | 4/468 | 6 (0.94%) | Maternal outcomes: anemia, preeclampsia, gestational diabetes and obstetric complications, such as abruptio placenta, overall rate of cesarean section, cesarean section for fetal distress, assisted vaginal delivery and postpartum hemorrhage. neonatal outcomes: low birth weight (LBW), prematurity, intrauterine growth restriction (IUGR), Apgar score at 1 min, NICU admission and fetal demise | - | Fair |
| Su (23) | China | Prospective population-based Cohort | TSH < 5th per., fT4 5th - 95th per. | 1017 | 31/845 | 31 (3.0%) | Birth weight; spontaneous abortion; fetal death; medically induced labor; malformations; fetal distress; preterm delivery; low birth weight; macrosomia; SGA; fetal stress, neonatal death, and infant development. | Maternal age, parity, and BMI | Good |
| Wilson (24) | USA | prospective population-based cohort | TSH < 0.03 mlU/L; normal fT4 levels | 24,883 | 584/23771 | 584 (2.3%) | Hypertension (gestational hypertension, mild preeclampsia, or severe preeclampsia) | Maternal age and weight, race , parity | Good |
| Ajmani (3) | India | Prospective cohort | TSH < 0.2, l U/L free T4 (0.8 - 2.0 ng/dL) | 400 | 3/347 | 0.75% | Maternal outcomes: anemia, PIH, abruptio placenta, cesarean section, postpartum hemorrhage. Fetal outcomes: birth weight, Apgar score at one and five minutes, NICU admission, preterm delivery, IUGR, fetal distress, and intrauterine demise. Neonatal outcomes: hyperbilirubinemia, respiratory distress, sepsis, hypoglycemia, hypothermia, intracranial bleed, necrotizing enterocolitis, and early neonatal death. | - | Fair |
| Saki (22) | Iran | Prospective cohort | TSH 0.1 - 0.2 mIU/L) with normal FT4 | 600 | 2/497 | 2 (0.3%) | preeclampsia, IUGR, preterm delivery and low Apgar score, cesarean section | Maternal age, maternal BMI, and preeclampsia | Good |
| Zhang (2) | China | Prospective cohort | TSH < 0.35 mIU/L, and 13.72 ≤ FT4 ≤ 20.22 pmol/L | 3,783 | 120/3573 | 120 | Abortion, gestational hypertension, eclampsia placenta previa, placental abruption, PROM, fetal macrosomia, LBW, breech position, premature delivery | - | Fair |
| Zhou (37) | China | Cohort | TSH < lower limit and fT4 within the normal range. | 2,676 | 9/513 | 9 | delivery Complications (gestational diabetes, gestational hypertension, intrahepatic cholestasis of pregnancy (ICP)) and adverse outcomes (premature birth, VLBW, LBW, birth asphyxia, fetal distress, PROM, placental abruption, miscarriage, fetal malformation, mortality) | - | Fair |
| Avramovska (38) | North Macedonia | Cohort | TSH < 0.1 mIU/L and TT4 level normal | 358 | 7/218 | 7 (1.94%) | Preterm births, IUGR, LBW, Apgar score (1 minute) < 7 | - | Fair |
Characteristics of Studies Included in the Meta-analysis
The included studies used a threshold of 0.03 to 0.5 mIU/L for diagnosing subclinical hyperthyroidism (Table 1). A total of 69,862 pregnant women participated in these studies, including 1,418 pregnant women with subclinical hyperthyroidism and 58,502 euthyroid ones. The pooled mean age and BMI (95% CI) of the study population were 26.6 (95% CI: 25.7, 27.4) years and 24.6 (95% CI: 21.7, 27.5) kg/m2, respectively.
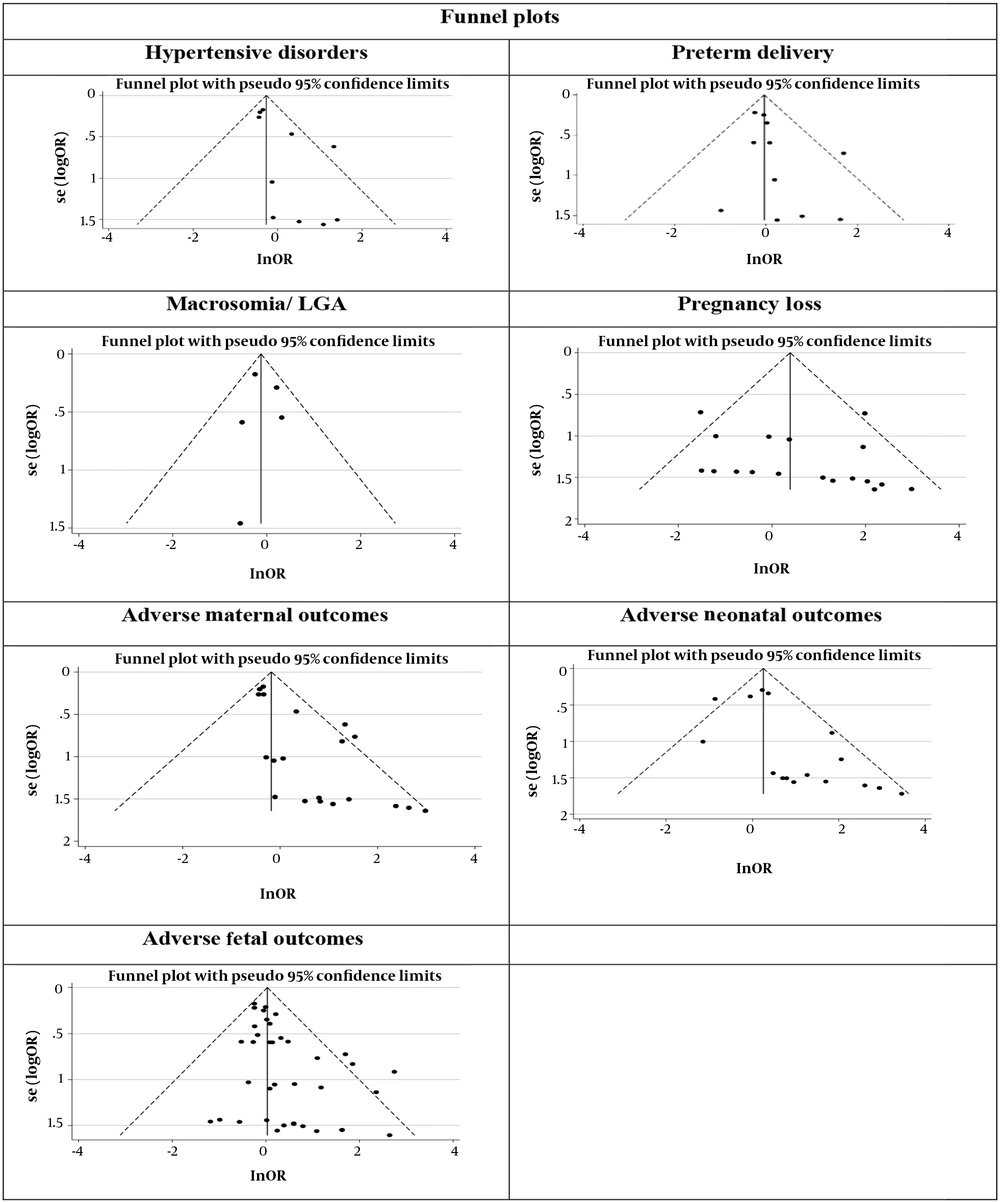
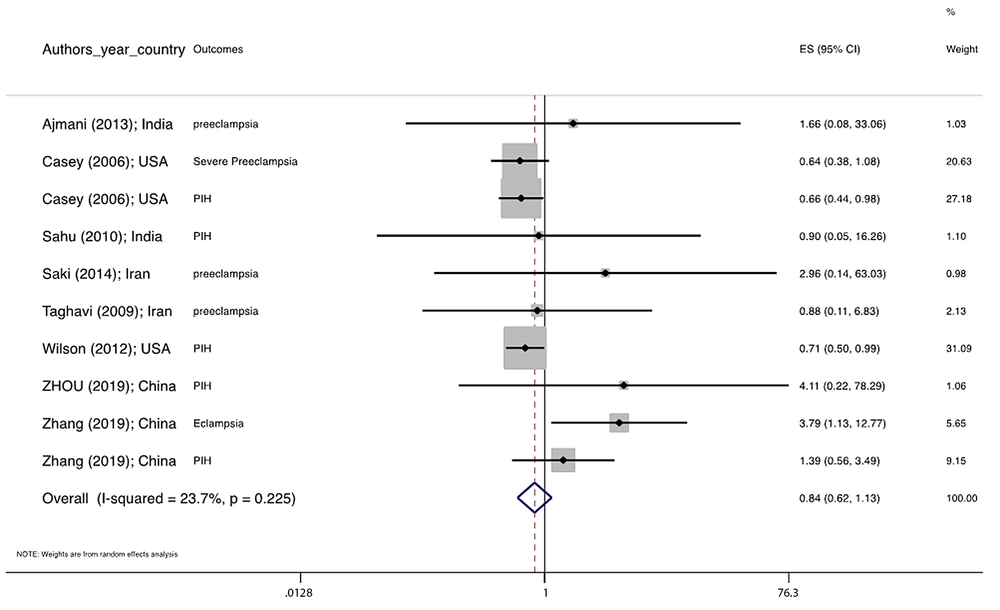
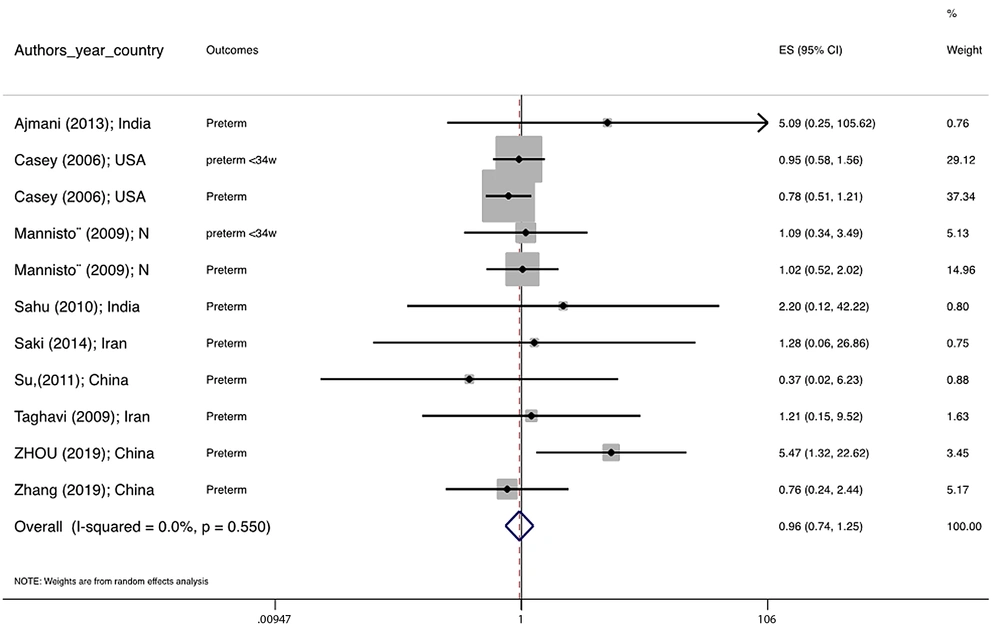
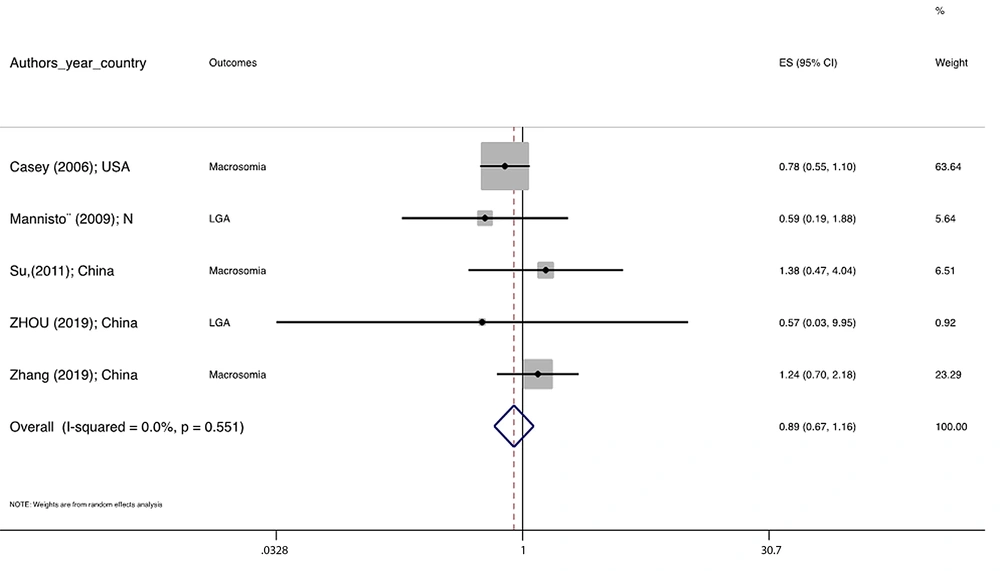
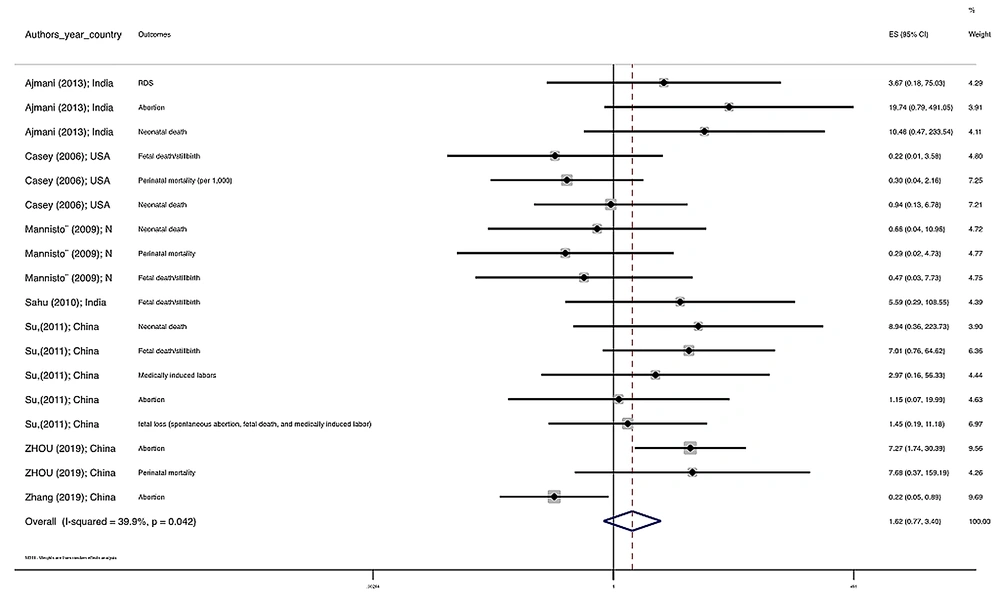
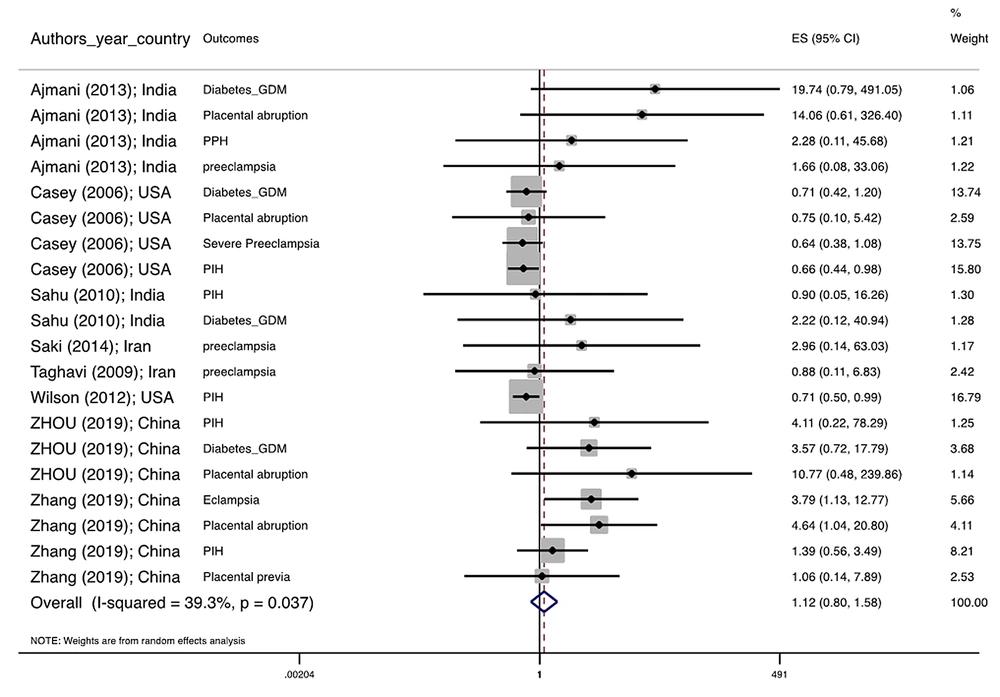
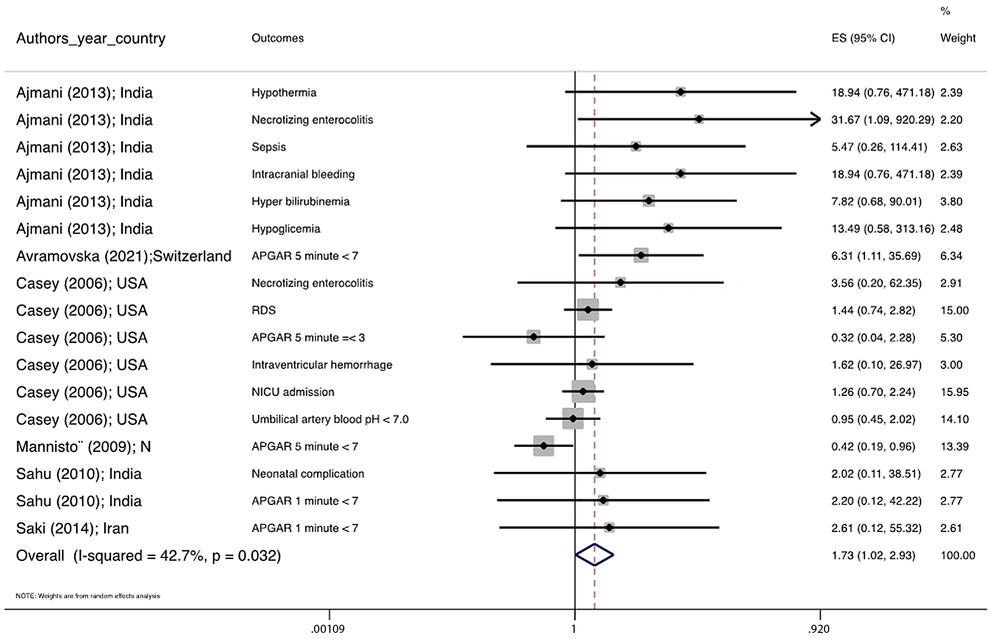
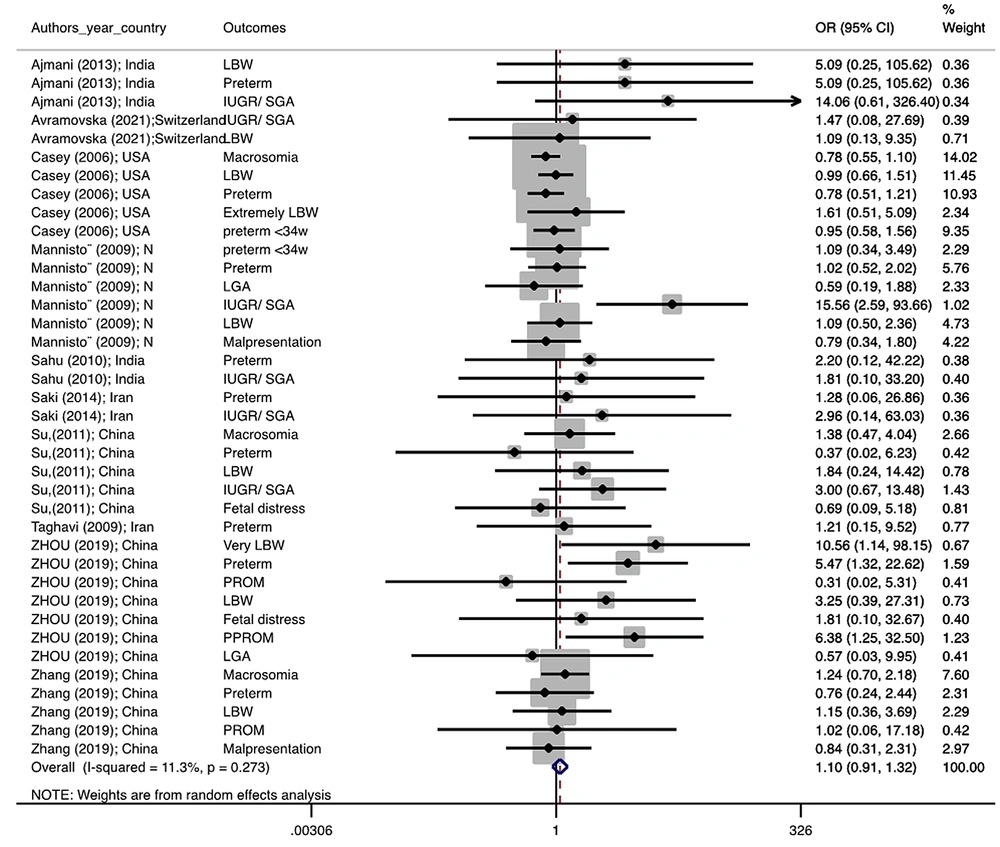
Figure 1 illustrates the flowchart of the search strategy and the study selection. Figure 2 illustrates the publication bias via funnel plot, bias was observed for various pregnancy outcomes including hypertensive disorders, adverse maternal outcomes, adverse neonatal outcomes, and adverse fetal outcomes. Figures 3 - 9 show the forest plots through random effect for pregnancy outcomes. Sensitivity analyses revealed no source of heterogeneity for any single study (Appendix 2).
4.2. Meta-analysis of Outcomes
Table 2 presents the results of the Egger test, I-squared %, pooled OR (95% CI), and pooled OR (95% CI) via trim and fill method for pregnancy outcomes in women with subclinical hyperthyroidism compared to euthyroid controls (with and without application of trim and fill method). No significant heterogenicity was detected among included studies investigating adverse pregnancy outcomes. Egger test showed significant publication biases only among studies investigating Hypertensive disorders (P = 0.031), adverse maternal outcomes (P = 0.001), adverse neonatal outcomes (P = 0.008), and adverse fetal outcomes (P = 0.001), which were adjusted by the trim and fill method.
| Outcomes | P-Value, Egger Test | I-squared% | Pooled Odds Ratio (95% CI) | Pooled Odds Ratio (95% CI) via Trim and Fill Method |
|---|---|---|---|---|
| Hypertensive disorders | 0.031 a | 33.0 | 0.839 0.615 1.143 | 0.684 0.466 1.002 |
| Preterm delivery | 0.144 | 0.0 | 0.963 0.739 1.253 | -- |
| Macrosomia/ LGA | 0.847 | 0.0 | 0.885 0.673 1.165 | -- |
| Pregnancy loss | 0.681 | 0.0 | 1.668 0.728 3.822 | -- |
| Adverse maternal outcomes | 0.001 a | 0.0 | 1.133 0.802 1.600 | 0.760, 0.514, 1.123 |
| Adverse neonatal outcomes | 0.008 a | 0.0 | 1.781 1.026 3.091 | 1.055, 0.597, 1.863 |
| Adverse fetal outcomes | 0.001 a | 0.0 | 1.095 0.911 1.317 | 0.953, 0.747, 1.215 |
Results of Meta-analysis for Pregnancy Outcomes in Women with Subclinical Hyperthyroidism Compared to Euthyroid Controls (With and Without Application of Trim and Fill Method)
There were no significant differences in pooled ORs of hypertensive disorders, preterm delivery, macrosomia/LGA, and pregnancy loss, in subclinical hyperthyroidism pregnant women, compared to the euthyroid controls group. Also, the pooled OR of adverse maternal and fetal outcomes had no statistically significant difference in subclinical hyperthyroidism compared to the euthyroid controls group with and without the trim and fill method. The Pooled OR of adverse neonatal outcomes in women with subclinical hyperthyroidism in comparison with euthyroid was 1.781 (95% CI: 1.026, 3.091); this statistically significant difference was disappeared using the trim and fill method (OR: 1.055, 95%CI: 0.597, 1.863) (Table 2).
4.3. Quality Assessment and Risk of Bias
The results of the quality assessment are accessible in Appendix 1; of 9 studies, 5 studies were classified as having good quality and 6 studies as fair quality. No studies did not identify as poor quality (Table 1 and Appendix 3).
Details of the risk of bias assessment are shown in Appendix 4. All cohort studies had a low risk of bias for selection of exposed and non-exposed cohorts, assessment of exposure, presence of outcome of interest at the start of study, outcome assessment, and adequacy of follow-up of cohorts; however, 44% of studies had a high risk of bias for control of prognostic variable and 11% for assessment of the presence or absence of prognostic factors; and 22% of studies were probable high risk of bias for assessment of the absence or presence of prognostic factors (Appendix 4). In general, most of the studies included in the meta-analysis had an acceptable validity (low risk of bias), which indicates the acceptable quality of these studies in most respects.
5. Discussion
Despite existing evidence on adverse outcomes in pregnant women with overt thyroid dysfunction (21, 40-43), it is unclear whether maternal subclinical hyperthyroidism is also associated with adverse pregnancy outcomes. The current meta-analysis showed that subclinical hyperthyroidism is not associated with any adverse maternal, neonatal, and fetal outcomes.
It is well-documented that maternal subclinical hyperthyroidism, a low serum TSH but normal serum T4 and T3 concentrations, is mainly caused by mild thyroid hormone excess, but this situation may have resulted from non-thyroidal illness, excessive consumption of levothyroxine, and ingestion of drugs that inhibit TSH secretion or it may be originated from inappropriate secretion of TRH or TSH (10, 11). Presumably, maternal subclinical hyperthyroidism is a transient dysfunction during pregnancy caused by placental hormone stimulation (44); as a result, these physiological changes need to be considered, rather than being interpreted as a pathological situation. It has been well-documented that hCG has a significant thyroid-stimulating activity, which can stimulate the thyroid gland under both in vivo and in vitro. Therefore, the thyroid may be controlled twice from both hCG and TSH at the early pregnancy stage (45). The increased hCG concentration at the early pregnancy stage is associated with an increase in free thyroid hormones and a decrease in serum TSH. Notably, HCG-related pregnancy serum activity also can stimulate FRTL-5 cells, which may account for some changes in thyroid function observed during pregnancy (45). There is evidence suggesting a clear mirror image between serum levels of hCG and TSH (46). Partial or total serum TSH suppression, in association with high serum hCG levels, is a frequent finding in normal pregnancy that usually occurs as a transient feature in the late first trimester of pregnancy (46). Consequently, it leads to transient subclinical hyperthyroidism in some women (47, 48); however, this TSH suppression does not usually result in hyperthyroidism symptoms (46). Korevaar et al. found that higher hCG concentrations were associated with a higher risk of subclinical and overt hyperthyroidism (49).
Few studies have investigated adverse pregnancy outcomes in women with subclinical hyperthyroidism, with contradictory results (2, 5, 12, 26, 27, 37). In the majority of the available studies, the number of pregnant women with subclinical hyperthyroidism is small. As a result, they had inadequate power for the assessment of adverse pregnancy outcomes in this subgroup of women (3, 9, 22, 23, 37). Three meta-analyses assessed the adverse pregnancy outcomes in women with subclinical hyperthyroidism. These studies had mainly focused only on preterm delivery, IUGR, and LBW and found no significant association between subclinical hyperthyroidism and these outcomes (25, 30, 50). This meta-analysis of nine studies demonstrated that subclinical hyperthyroidism was not associated with adverse maternal outcomes, such as hypertensive disorders, preterm delivery, and pregnancy loss, neonatal outcomes, such as macrosomia/LGA, and adverse fetal outcomes. The lack of the association between subclinical hyperthyroidism and adverse pregnancy outcomes may be mainly explained by these physiological changes in thyroid hormones during pregnancy. Actually, the majority of these women had transient gestational hyperthyroidism, which might consider a physiologic condition related to pregnancy. Although there is evidence demonstrating adverse effects of excessive thyroid hormones and subclinical hyperthyroidism on the cardiovascular system in terms of increasing heart rate, carotid intima-media thickness, plasma fibrinogen levels, left ventricular mass atrial arrhythmias, impaired ventricular relaxation, and reduced exercise performance (51-55), this study showed no significant relationship between this mild maternal thyroid dysfunction and gestational hypertensive disorders.
To the best of our knowledge, this study is one of the few meta-analyses to evaluate the association between subclinical hyperthyroidism during pregnancy and maternal, neonatal, and fetal outcomes. However, this study has several limitations that should be considered for interpreting its findings.
Firstly, various cut-off points were used to diagnose subclinical hyperthyroidism in different studies, which may affect the observed outcomes. Secondly, a limited number of publications evaluated the impact of subclinical hyperthyroidism on pregnancy outcomes compared to euthyroid pregnant women. Third, the majority of studies have not adequate statistical power due to the small number of included pregnant women with subclinical hyperthyroidism as well as the small number of adverse pregnancy outcomes. Fourth, the lack of adequate statistical power for some pregnancy outcomes limited us from running analysis for these outcomes. Fifth, TPOAb status was not determined in most of the included studies, and in studies where thyroid antibodies were measured, results were not compared to the pregnancy outcomes in subgroups of subclinical hyperthyroidism based on the TPOAb status (positive and negative) and their results were not adjusted for TPOAb status. Sixth, the iodine status and iodine supplementation have not been reported and the results were not adjusted for these variables.
Finally, the lack of adequate well-designed studies and adequate power for subgroup analysis according to the type of study or gestational age. Further studies with a large sample size among euthyroid pregnant women are necessary.
5.1. Conclusions
The current meta-analysis demonstrated that maternal subclinical hyperthyroidism is not associated with adverse maternal, neonatal, and fetal outcomes. Therefore, clinicians should be avoided unnecessary treatments for pregnant women with subclinical hyperthyroidism. However, more longitudinal studies of women with subclinical hyperthyroidism with adequate power are recommended for precise interpretation of the association between this mild thyroid dysfunction and adverse pregnancy outcomes after adjusting potential confounders.