1. Introduction
Osteoporosis-pseudoglioma syndrome (OPPG) is an autosomal recessive disorder characterized by severe osteoporosis in childhood and congenital or juvenile-onset ocular defects (1). The patients are predisposed to recurrent long bone fractures, deformities due to muscle weakness such as kyphoscoliosis, vertebral compression fractures, extremity deformities, and short stature in adulthood (2). The cortical bone is thinned, and the metaphyses are enlarged, with severe decrease in bone density. Visual impairment in these patients includes a wide range of ocular disorders, and most affected children are blind congenitally or become blind at the beginning of their childhood (3).
OPPG is caused by biallelic loss-of-function mutation in the low-density lipoprotein receptor-related protein 5 (LRP5) gene, which is located at 11q (4). It is assumed that the activation of LRP5 by Wnt and/or blocking Dkk1 has primary role in increasing bone formation and reducing bone resorption. The polymorphism of exon 18 of LRP5 gene is associated with vertebral bone mineral density (BMD) and final height in children (3).
Several medications have been developed to treat osteoporosis, including raloxifene, bisphosphonates, denosumab, abaloparatide, and teriparatide. The molecular mechanism of bisphosphonate is substantially understood. Bisphosphonates reduce bone turnover and prevent osteoclast function through inhibition of the mevalonate pathway, which leads to reduced bone resorption. Teriparatide (recombinant human parathyroid hormone 1 - 34) is an anabolic agent indicated in the treatment of osteoporosis that increases the osteoblasts activity and inhibits the osteoblasts apoptosis. Teriparatide is known to markedly increase BMD in the lumbar spine and femur hip in OPPG without any relevant adverse events (5).
In present study, we present an Iranian girl with OPPG and report the results of her treatment with teriparatide. To the best of our knowledge, this is the first experience of treatment with teriparatide in an adolescent with OPPG before epiphyseal closure.
2. Case Presentation
The patient was a 17-year-old Iranian girl with OPPG born of a consanguineous marriage with no history of OPPG in parents. The patient was born as full-term by cesarean section, and she was the second child of the family. Her birth weight, height, and head circumference were 3.1 kg, 52 cm, and 35 cm, respectively. Soon after birth, the parents noticed her visual impairment. The ophthalmologist identified high intra-ocular pressure, bilateral microphthalmia with corneal opacities, congenital phthisis bulbi, and retinal detachments.
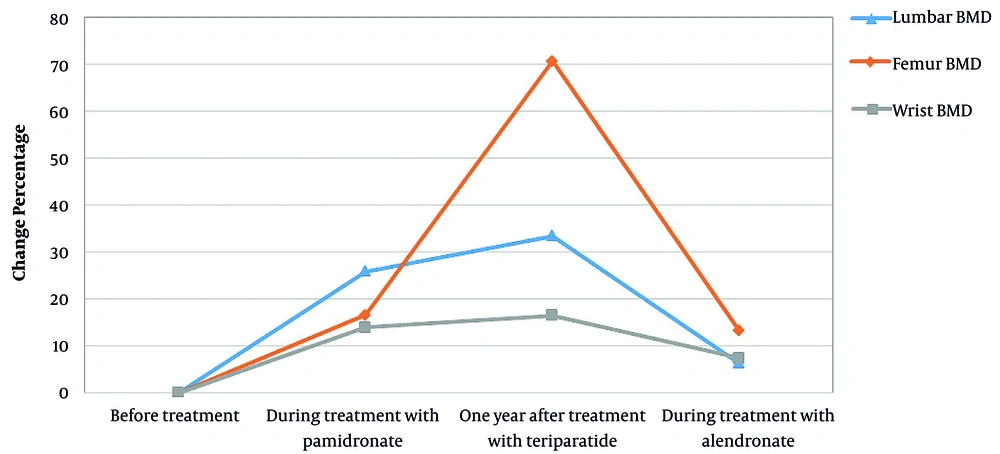
At the age of two years and during occupational therapy due to developmental delay, she experienced a fracture in her right femur. One year later, another fracture occurred on her wrist as she fell off a bike. After examination, she was diagnosed with OPPG and treatment with pamidronate was initiated with 1 mg/kg daily infusions for three days every three months up to age 11 years and six months. Despite several years of treatment with pamidronate, she had multiple bone fractures. During treatment with pamidronate, BMD was assessed twice (eight years and three months, ten years and six months), the results of which are shown in Figure 1. The BMD was performed by absorptiometry method (DEXA) and HOLOGIC machine (model: discovery W S/N 83407).
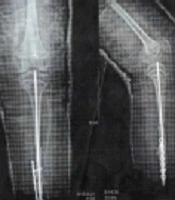
The patient had multiple tibial fractures without any obvious trauma in earlier years of life. The fractures were fixed using internal fixators, but the patient was bedridden, and further hip fractures happened despite several years of bisphosphonate therapy (Figure 2). The patient had autism and was unable to communicate or attend school. At age 11 and during treatment with pamidronate, while both upper limbs were in plaster due to previous fractures, the patient fell over again while using toilet and got multiple fractures in femur and pelvic bones. At this time, liver, kidney, and thyroid function tests, calcium, phosphorus, and parathyroid hormone (PTH) levels were normal, and only vitamin D levels were below normal (15 ng/mL; normal range > 30 ng/mL).
Due to the patient's poor physical condition, disability, a lack of response to conventional pamidronate therapy (as mentioned above), and given the patient's critical condition, a course of treatment with teriparatide was initiated at the age of 11 years and nine months; however, her epiphyseal plate was not closed. We had no other options but to start teriparatide while informing the mother and receiving a consent letter. Due to the openness of the growth plates, we prescribed a short period of teriparatide to prevent possible side effects.
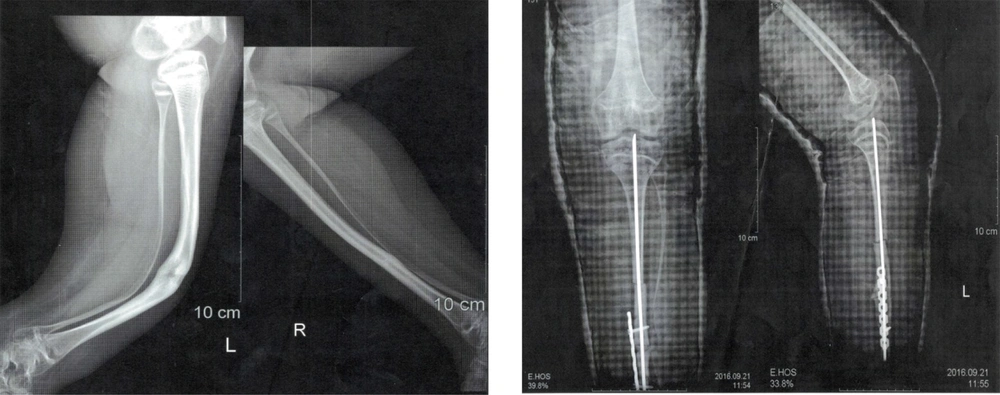
The patient received teriparatide (Forteo, Eli Lilly, Indianapolis, IN, USA) 20 µg daily (the same standard dose in adults) by subcutaneous injection during a four-month period sequentially. The patient was receiving 500 mg oral calcium and vitamin D 1000 IU concurrently, followed by 35 mg oral alendronate once a week. The results of the BMD showed significant improvement, and she became ambulant. One year after treatment with teriparatide, fractures were completely healed, and the patient was able to walk independently. One year after teriparatide treatment, BMD increased by 33.5% in the lumbar region and 70.7% in the femoral region (Figure 2). Routine test outcomes, electrolytes, lipids, and vitamin D levels were normal during and after treatment with teriparatide, and no side effects have been observed so far. One year after receiving the last dose of teriparatide, the treatment with oral alendronate (35 mg) weekly began, which is still ongoing. Since then, she has not had a broken bone.
The results of bone densitometry measurements before and after treatment are presented in Figure 2. Current weight and height are 54 kg and 134 cm. Menarche was at age 12 years and it was regular thereafter. The height of the mother was 167 cm, and the father’s height was reported 174 cm by the mother.
His older brother also had OPPG. He was blind and had severe osteoporosis, but by the age of 26 he had no bone fractures. Genetic testing was performed for the patient and her mother, and a novel homozygous nonsense mutation (c.351G>A) in exon 2 of the LRP5 gene was reported.
3. Discussion
The patient had severe osteoporosis and recurrent bone fractures and was unable to move, but after receiving a four-month period of teriparatide treatment, she was able to walk independently. OPPG patients have severe bone formation deficiency from the beginning of the bone tissue formation, which represents low bone mass density (4). The patient's final height was less than the average height calculated according to the heights of her parents, which could be due to the underlying disease, multiple bone fractures in childhood, and osteoporosis. Intellectual disability, obesity, and muscular hypotonia are clinical features that can be identified in affected individuals with OPPG (6). Our patient was unable to communicate, and she was diagnosed with autism.
Although the patient had been treated with pamidronate for several years, no clinical improvements were observed, bone fractures continued, there was tenderness in touch of the bones, and she was unable to walk independently.
Several studies have assessed the effects of bisphosphonate therapy on OPPG patients (7-9). Tallapaka et al. reported the beneficial effects of bisphosphonate therapy resulting in increased bone mineral density, decreased bone fracture rate, and therefore improved quality of life (7). However, some other studies reported that bisphosphonate therapy did not have many effects (9-11). Our study also confirms the low effect of pamidronate on improving bone density and reducing bone fracture rate in OPPG. Streeten et al. reported significant increase in the areal BMD in OPPGS patients after treatment with bisphosphonate, but trabecular volumetric BMD remained low. As a result, during long-term follow-up, there was no significant improvement in bone fractures (12), which was similar to the findings of our study. However, bisphosphonates cannot be considered as an ideal treatment for treatment of OPPG in some patients. Streeten et al. reported fractures in three of nine OPPG patients with low-normal hip areal BMD after treatment with bisphosphonates (12). Our patient had multiple bone fractures after about seven years of pamidronate treatment. Also, her BMD in the lumbar region increased slightly (from 0.361 gr/cm2 to 0.532 gr/cm2) but did not change much in the femoral (from 0.302 gr/cm2 to 0.372 gr/cm2) and wrist (from 0.410gr/cm2 to 0.463gr/cm2) areas. Arantes et al. reported a 12-year-old boy with OPPG. In their study, the patient was firstly treated with pamidronate for six years, and BMD was increased in the first three years of treatment. The teriparatide therapy was initiated one year after the pamidronate treatment was ended, resulting in markedly improvement of BMD without any complication after two years of follow-up (13).
The patient we studied was in poor physical condition and did not respond to pamidronate and conventional maintenance treatments, but 12 months after treatment with teriparatide, her physical condition significantly improved, and her BMD was dramatically increased (from 0.532 to 0.711gr/cm2 in spine and 0.372 to 0.635 gr/cm2 in femur neck). She did not have any fractures and other complications after treatment with teriparatide until now. Administration of teriparatide results in increased bone formation, improved bone strength, and bone mass. Continuous administration of PTH increases bone resorbing osteoclasts, while intermittent administration increases bone forming osteoblasts, improves BMD, and reduces fracture risks (14). Mild hypercalcemia is one of the complications of treatment with teriparatide, with an incidence of 1 - 3%. There is no evidence for increased risk of osteosarcoma in humans (15).
3.1. Conclusions
In children and adolescents diagnosed with OPPG who do not respond to other conventional therapies, short courses of teriparatide therapy may be helpful.
3.2. Limitations
We did not measure bone markers such as CTX and P1NP in this study.