1. Introduction
Malignant sellar gliomas are very rare entities. To date, there have been only few reports of sellar and suprasellar glioblastomas. Here, we present a clinical case of endo-suprasellar glioblastoma, which was initially classified as a macroprolactinoma.
2. Case Presentation
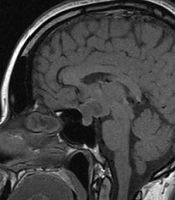
A 34-year-old woman reported a one-year history of amenorrhea and headache. She had no complaints of galactorrhoea or other symptoms. Primary laboratory tests revealed hyperprolactinemia of 140 ng/mL (Table 1). Contrast-enhanced magnetic resonance imaging (MRI) was performed for the patient, which showed a mass lesion in the pituitary stalk, extending to the chiasm and adenohypophysis (14 × 12 × 12 mm) (Figure 1). The lesion was isointense on T1-weighted images compared to the adjacent grey matter and demonstrated bright homogenous enhancement after contrast administration. Hormone tests excluded the overproduction of adrenocorticotropic hormone (ACTH), growth hormone (GH), and thyroid-stimulating hormone (TSH) by the tumor (Table 1) and indicated secondary hypogonadism.
| Laboratory Tests | Results | Reference Range |
|---|---|---|
| Prolactin, ng/mL | 140 | < 29.9 |
| Macroprolactin, % | < 20 | |
| Thyroid-stimulating hormone, mU/L | 1.87 | 0.4 - 4.0 |
| Free thyroxine, pmol/L | 9.45 | 9.0 - 19.0 |
| Somatomedin-C (IGF-1), ng/mL | 170 | 125 - 311 |
| ACTH, pg/mL | 23 | 0 - 46 |
| Cortisol, nmol/L | 253 | 101.2 - 535.7 |
| Late-night salivary cortisol, nmol/L | < 1.5 | < 9.4 |
| Luteinizing hormone, mIU/mL | < 0.09 | 1.68 - 15.0 |
| Follicle-stimulating hormone, mIU/mL | 0.25 | 1.37 - 9.9 |
| Estradiol, pmol/L | 140 | 68 - 1269 |
Primary Hormone Examination
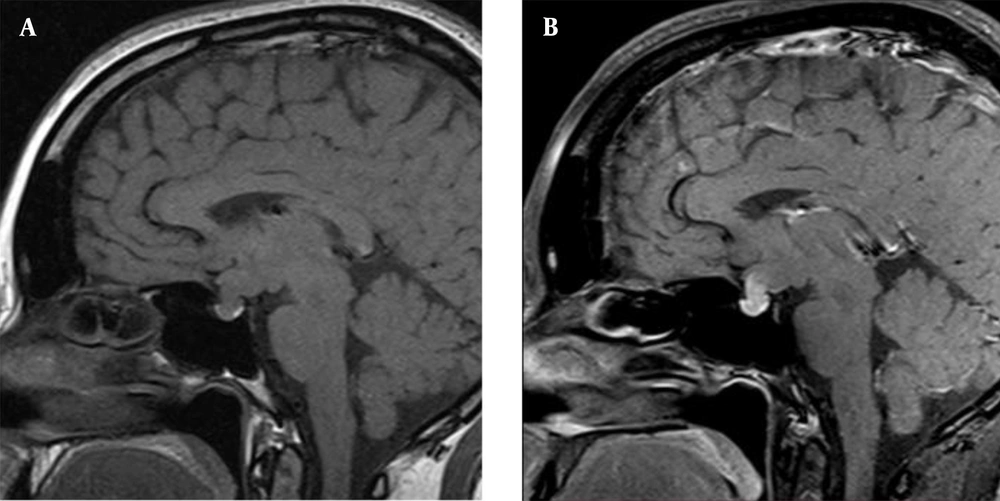
The perimetry did not show any visual field impairments. The prolactin test was not repeated after serum sample dilution during the initial examination at the outpatient hospital. Despite the discrepancy between the tumor size and the prolactin level, the hook effect was not excluded, and the mass was regarded as a macroprolactinoma. The patient was prescribed cabergoline (0.25 mg per week), which significantly reduced the level of prolactin below the reference range (< 0.6 ng/mL); however, it did not lead to the recovery of menstrual function. Cabergoline was subsequently discontinued, and the patient was asked to return for a follow-up visit within one month. However, she was lost to follow-up and did not return to the hospital at the appointed time. Few months later, she noticed deterioration of eyesight, blurred vision, and narrowing of the visual field in both eyes. Accordingly, she scheduled an appointment at the Endocrinology Clinic of I.M. Sechenov First Moscow State Medical University. The follow-up non-contrast MRI study (nine months after the primary MRI) indicated deterioration of the patient’s condition. The mass had increased in size up to 20 × 21 × 23 mm, and the pituitary stalk was significantly enlarged (Figure 2).
The T1-weighted images indicated a hypointense zone with numerous cystic transformations at the center of the lesion, probably due to central necrosis. The lesion had no distinct boundaries and was indistinguishable from the pituitary stalk. The tumor was spreading into the suprasellar cistern, squeezing and displacing upward the chiasm, and pushing the anterior cerebral arteries outwards. The prolactin level was 132 ng/mL. The ophthalmological examination revealed partial optic nerve atrophy, retinal angiopathy, and heteronymous bitemporal hemianopsia.
Considering the continuous tumor growth and progression of sellar syndrome, the patient underwent a transnasal removal of the sellar mass. According to further histological and immunohistochemical analyses, a malignant glioma with polymorphism of cells and nuclei, mitoses, and pronounced proliferation of vascular endothelium was confirmed. The immunohistochemical study revealed the positive expression of glial fibrillary acidic protein (GFAP), as well as S100 expression in the tumor and CD34 expression in the vascular endothelium. The Ki-67 value for the tumor was measured to be 15%. Therefore, a diagnosis of high-grade malignant glioma (HGG) was confirmed, and tumor growth from the pituitary funnel or neurohypophysis could not be ruled out.
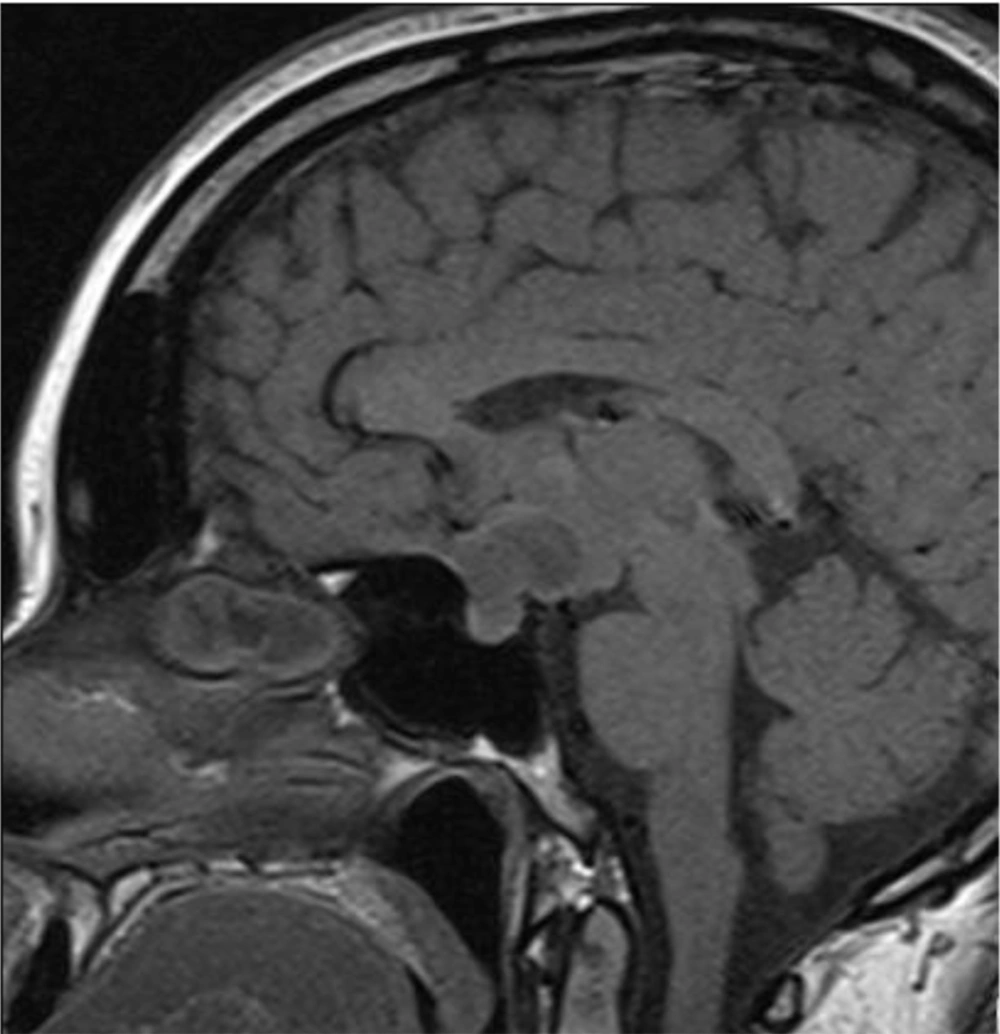
The patient’s vision remarkably improved after surgery. However, she developed diabetes insipidus and panhypopituitarism (secondary hypothyroidism, secondary hypocortisolism, and secondary hypogonadism), requiring chronic hormone replacement. Postoperative contrast-enhanced MRI showed signs of residual tumor in the sellar region up to 9 × 11 mm, with inhomogeneous contrast enhancement (Figure 3). The patient was prescribed a chemoradiation therapy (56 Gy, 140 mg of temozolomide per day during the entire period of radiation therapy, followed by 340 mg of temozolomide per day for five days with a break of 23 days in 12 cycles).
3. Discussion
Pituitary adenomas, originating from the anterior part of the pituitary gland, are the most common tumors in the sellar region, followed by craniopharyngiomas and Rathke cleft cysts and less commonly, meningiomas, germinomas, hamartomas, and metastatic tumors. In rare cases, a chiasmatic-sellar mass may originate from either the glial tissue, involving the optic tract and pituitary stalk, or pituicytes (neurohypophysis cells), which originate from ependymal cells (1-3). Most of these tumors (gliomas, astrocytomas, and pituicytomas) are associated with low-grade malignancy and slow growth. Therefore, the treatment plan may not differ from that of hormonally inactive pituitary adenomas.
On the contrary, malignant sellar gliomas are very rare, and only few cases of suprasellar malignant gliomas have been reported (4-9). We found only five cases of endo-suprasellar malignant gliomas, originating from the hypothalamic/pituitary axis, which could be initially mistaken for pituitary macroadenomas (4-7). Overall, considering the high malignancy of glioblastomas, it is important to inform clinicians and radiologists about these tumors in the chiasmatic-sellar region. Besides, it is essential to discuss the possibility of differential diagnosis during the preoperative stage, which can be useful for early selection of a treatment plan.
However, MRI examination of pituitary tumors does not clearly differentiate the most common adenomas from gliomas and glioblastomas of the pituitary gland, as both types of lesions (similar to the clinical case described here) can be hyperintense on T2-weighted images and iso- or hypo-intense on T1-weighted images, with features of central necrosis in tumors (4-9). Moreover, squamous papillary craniopharyngiomas, which are more common in adults, may have similar MRI characteristics; this also complicates the differential diagnosis of these masses (10). In the present case, there were signs of restricted diffusion on the brain MRI by the pituitary gland lesion. However, this finding could not help us establish an accurate diagnosis, because it is well-known that macroadenomas with soft consistency have a high signal intensity on diffusion-weighted imaging (DWI), with a low apparent diffusion coefficient (ADC) (11).
Rapid tumor growth, unrelated to hormonal abnormalities (in the absence of hormonal activity of tumor), may lead to a suspicion of glioblastoma in the sellar region. In the present case, an endo-suprasellar mass, originating from the pituitary gland, was initially mistaken for a macroprolactinoma due to moderate hyperprolactinemia. The observed discrepancy between the tumor size and slightly elevated prolactin levels may be a result of the hook effect. Therefore, the prolactin test should have been repeated following serum sample dilution; this could have distinguished between a prolactinoma and a non-functioning adenoma. This test was not repeated for our patient, because her initial examination was performed at an outpatient hospital. Nevertheless, the significant decrease in the prolactin level below the defined range with minimal doses of cabergoline suggested that hyperprolactinemia was a secondary condition and indicated the development of a sellar tumor.
Besides, the primary MRI showed that the tumor originated from the pituitary stalk, which is not common in prolactinomas. Continuous tumor growth during cabergoline therapy revealed that the mass was not a prolactinoma; the rapid progression of tumor growth led to a suspicion of high-grade malignancy. In previously published clinical studies of endo-suprasellar malignant gliomas originating from the hypothalamic/pituitary axis, the clinical manifestations of tumors included acute neurological symptoms (vomiting and headache) (5, 7), visual disorders (4-6), endocrine disorders (amenorrhea, galactorrhea, and diabetes insipidus) (4, 7), loss of appetite, and fatigue (4). Also, more invasive and larger tumors were detected in the primary MRI compared to our case. Nonetheless, similar to the present case, the MRI images did not indicate any specific features to allow differentiation of malignant gliomas from other more common chiasmatic-sellar tumors. In two published cases, the laboratory examinations revealed that the serum prolactin levels were two to three times higher than the reference range (4, 7); besides, Hypogonadotropic hypogonadism and panhypopituitarism were also reported (4). There were no pituitary function disorders in other cases. Overall, these features may help clinicians make a faster decision on the surgical treatment and use of a transcranial approach.
To clarify the histogenesis of malignant gliomas, an immunohistochemical analysis of the tumor can determine the expression of specific tumor proteins, including GFAP and S100 protein. The S100 protein is implicated in the regulation of cellular metabolism, motility, and proliferation. Under pathological tumor and inflammatory conditions, the concentration of S100 protein increases, which stimulates microglia and astrocytes and increases the expression of pro-inflammatory cytokines (12). GFAP is the most widely used marker of astrocytes. During injuries (trauma or disease), the expression of GFAP in astrocytes rapidly increases. GFAP is often used to indicate the astrocytic lineage of glial cells and glial tumor cells. It also plays a more significant role in tumor pathology than in the differential diagnosis of astrocytoma (12).
In this clinical case report, an immunohistochemical analysis of the removed tumor specimen revealed the positive expression of GFAP and S100, which confirmed the diagnosis of HGG, as it was accompanied by the results of histological analysis and a high Ki-67 index (15%). In other cases of endo-suprasellar malignant gliomas described above, the most common immunohistochemical marker was GFAP, and the Ki-67 index ranged from 15% to 60% (4-7). An additional marker for high malignancy is the expression of a transmembrane phosphoglycoprotein (i.e., CD34) in the vascular endothelium, since its overexpression is associated with higher WHO grades of gliomas (13). Besides, CD34 may serve as a potential diagnostic and prognostic marker or an effective therapy target (13).
In the majority of published cases, chiasmatic glioblastomas in the sellar region originated from the optic nerve/optic chiasm (8, 9). The differential diagnosis of glioblastomas of the optic tract and hypothalamic/pituitary glioblastomas may be difficult, especially in the advanced stages of disease, with an extensive sellar syndrome, involving visual disorders, hypopituitarism, and hyperprolactinemia. Considering their common origin, these tumors do not differ in terms of their histological and immunohistochemical characteristics. Consequently, they can be only distinguished based on the clinical symptoms and MRI findings. In our case, the tumor clinically manifested as amenorrhea and was accompanied by hyperprolactinemia and secondary hypogonadism, without visual impairments. MRI revealed an endo-suprasellar tumor, originating from the pituitary stalk. As the disease progressed, the emergence and growth of chiasmal syndrome was observed, which was confirmed by MRI, indicating continuous suprasellar tumor growth.
Malignant sellar gliomas are characterized by rapid and aggressive tumor growth and poor prognosis. Among five cases of hypothalamic/pituitary malignant gliomas described above, three resulted in the death of patients within a short period due to tumor progression or complications of surgical treatment (4, 6, 7). The outcomes of the other two patients were not reported (4, 5). It should be noted that in our case, the patient is currently under chemotherapy. Given the inadequacy of findings on the described type of tumor, it is difficult to confirm factors that determine prognosis. In particular, the prognostic value of Ki-67 is not completely clear. It has been suggested that Ki-67 predominantly affects the pattern of failure in HGG patients treated with a multimodal approach (14). Other possible factors that determine the prognosis of treatment include early diagnosis of tumor and radical resection during the primary surgery.
In conclusion, malignant sellar gliomas are very rare entities, which can be mistaken for macroadenomas or other more common chiasmatic-sellar tumors. Although they can mimic the clinical, endocrinological, and radiological features of pituitary macroadenomas, rapid progression without appropriate hormonal activity suggests their diagnosis.