1. Background
Pheochromocytomas are catecholamine-secreting neoplasms arising from chromaffin cells of the adrenal medulla, and paraganglioma are defined as tumors arising from extra-adrenal chromaffin tissue. These catecholamine-secreting neoplasms are rare, and the incidence of pheochromocytoma and paraganglioma (PPGL) is about 0.6 cases per 100,000 person-years (1). Excessive catecholamine secretion causes various symptoms, including hypertension and the classic triad of palpitation (tachycardia), headache, and sweating (2). It also presents comorbidities of diabetes mellitus (DM) or cardiovascular events.
Complete surgical resection is the definitive treatment for PPGL. However, unavoidable intraoperative manipulation of the tumor can cause catecholamine release and hypertensive crises. Therefore, surgery for PPGL has long been considered a challenging procedure (3). In the 21st century, the mortality associated with surgery for PPGL has dramatically decreased to a rate of 0% to 3.0%, which has resulted from improvements in treatment, such as surgical techniques, preoperative management including α-receptor blockades, and anesthesia (2). Regardless of these clinical efforts, surgery for PPGL can lead to intraoperative hypertensive crises, which cause life-threatening complications (4).
Although the guidelines issued by the Endocrine Society recommend preoperative α-receptor blockades as a prophylactic for all patients (5), a recent observational study showed that there was only a slight difference in maximum arterial pressure between patients who had been administered α-receptor blockades and those who had not (6). These results are important because not only might there be asymptomatic patients who do not need preoperative α-receptor blockades but also severe patients who experience intraoperative hypertensive episodes despite adequate doses of preoperative α-receptor blockades.
Intraoperative hemodynamic instability may occur in patients with PPGL despite adequate pretreatment (7). Recent studies have shown that some preoperative factors, such as symptomatic high blood pressure (BP), urinary-fractionated catecholamine metabolites, tumor size, and DM, were associated with hemodynamic instability during PPGL surgery (8, 9). However, the association between preoperative factors and intraoperative maximum arterial pressure is not well understood in PPGL patients who have received adequate pretreatment.
2. Objectives
In the current study, we retrospectively reviewed the clinical and operative records of patients with PPGL. Using this data set, we identified preoperative factors associated with intraoperative maximum arterial pressure for continuous variables in patients with pheochromocytoma who received adequate doses of preoperative α-receptor blockades.
3. Methods
3.1. Patient Selection
The medical records of 61 patients with PPGL who underwent surgical resection at Kansai Medical University Hospital between September 2006 and September 2020 were retrospectively reviewed. Pediatric and pregnant patients and those who received partial adrenalectomies (adrenal-sparing surgeries) were not included in the analysis. All patients enrolled were identified as having pheochromocytoma from the pathological specimens. The present study was approved by the institutional review board of Kansai Medical University Hospital (Approval No. 2015607).
3.2. Clinical Parameters
The preoperative clinical data (sex, age, BMI, American Society of Anesthesiologists (ASA) score, comorbidity, PPGL-associated classic triad, tumor size, and 24-h urinary-fractionated metanephrine (MN) and normetanephrine (NMN) were analyzed. The ASA score was used to assess a patient’s medical co-morbidities before anesthesia (ASA I is a normal healthy patient, ASA II is a patient with mild systemic disease, and ASA III is patient with severe systemic disease.). Clavien-Dindo classification was used to assess surgical complications (Grade III is complications requiring any interventions, Grade IV is life-threatening complications, and Grade V is death of a patient). Comorbidities included cerebro-cardiovascular events, hypertension (HTN), and DM. HTN was diagnosed as a systolic BP of 140 mmHg or higher or diastolic BP of 90 mmHg or higher without antihypertensive drugs at the time of medical examination. HTN was also defined as patients taking antihypertensive drugs. DM was defined as patients diagnosed by a diabetologist according to guidelines or taking DM-related drugs or insulin treatments. One or more of the classic triad was diagnosed in patients exhibiting at least one of the following symptoms: palpitation, headache, and episode of sweating. The maximal tumor size was measured by preoperative computed tomography (CT) or magnetic resonance imaging (MRI). These clinical data were obtained from the patients’ medical records.
3.3. Preoperative Management, Surgery, and Anesthesia
All patients were treated with the maximum dose of doxazosin (DOX) (16 mg/day) at least 2 weeks before surgery. Patients were admitted to the hospital the day before surgery, and preoperative systolic and diastolic blood pressure was measured at rest during the daytime. Retroperitoneoscopic and laparoscopic surgical techniques have been described in detail (10). Intraoperatively, the arterial pressure and heart rate were automatically recorded with an arterial line. Intravenous vasodilators, such as sodium nitroprusside, were administered to control undesirable increases in systolic arterial pressure (AP). All procedures were performed by experienced surgeons and anesthesiologists at a single institution.
3.4. Statistical Analysis
The primary outcome of this study was intraoperative maximum AP for continuous variables. It was defined as the highest arterial pressure during surgery, excluding the time of intubation and extubation. All continuous data are shown as median values and interquartile ranges (IQRs). The normal distribution of the results was confirmed by the Kolmogorov-Smirnov test. The logarithmic representation of continuous variables was calculated with base 10. Simple and multiple linear regression analyses were performed to evaluate the relationship between intraoperative maximum APs and preoperative variables. A reduced model selection was performed using a backward step-down selection process in the multiple linear regression analysis. In linear regression analysis, the t statistic, which was calculated as the ratio of an estimated coefficient (β) to its standard error (SE), was used to test the hypothesis that a coefficient was equal to zero. All statistical analyses were performed using EZR version 1.37 (11). A two-sided P value < 0.05 was considered statistically significant.
4. Results
4.1. Patient Characteristics
The clinical and tumor characteristics of the PPGL patients are shown in Table 1. The median intraoperative maximum AP was 165 mmHg (IQR: 150 - 180 mmHg). Of 61 patients, 18 (29.5%) had hypertensive episodes with intraoperative maximum APs above 180 mmHg. Preoperative hypertension and DM were shown in 28 (45.9%) and 6 (9.8%) patients, respectively. There were 16 (26.2%) patients exhibiting one or more features of the classic triad. No postoperative complications greater than grade 3 by the Clavien-Dindo classification were observed.
| Variables | Values (n = 61) |
|---|---|
| Age (y); median (IQR) | 54.0 (44.0 - 65.0) |
| Sex | |
| Female | 32 (52.5) |
| Male | 29 (47.5) |
| BMI (kg/m2); median (IQR) | 21.4 (19.7 - 24.8) |
| ASA | |
| I | 1 (1.6) |
| II | 59 (96.7) |
| III | 1 (1.6) |
| Cerebro-cardiovascular events | |
| Absent | 58 (95.1) |
| Present | 3 (4.9) |
| Hypertension | |
| Absent | 33 (54.1) |
| Present | 28 (45.9) |
| Diabetes mellitus | |
| Absent | 55 (90.2) |
| Present | 6 (9.8) |
| One or more of the classic triad b | |
| Absent (none of the symptoms) | 45 (73.8) |
| Present (at least one of the symptoms) | 16 (26.2) |
| Hereditary | |
| Unknown | 54 (88.5) |
| MEN | 5 (8.2) |
| VHL | 1 (1.6) |
| SDH | 1 (1.6) |
| Tumor location | |
| Pheochromocytoma | 52 (85.2) |
| Paraganglioma | 9 (14.8) |
| Type of surgery | |
| Laparoscopic | 51 (83.6) |
| Open | 10 (16.4) |
| Surgical approach | |
| Transperitoneal | 54 (88.5) |
| Retroperitoneal | 7 (11.5) |
| Tumor size (mm); median (IQR) | 38.0 (28.0 - 52.0) |
| Urinary-fractionated MN in 24-h urine (mg/day); median (IQR) | 0.32 (0.14 - 0.97) |
| Urinary-fractionated NMN in 24-h urine (mg/day); median (IQR) | 1.40 (0.65 - 3.60) |
| Preoperative systolic blood pressure (mmHg); median (IQR) | 120 (112 - 130) |
| Preoperative diastolic blood pressure (mmHg); median (IQR) | 72 (67 - 81) |
| Intraoperative maximum arterial pressure (mmHg); median (IQR) | 165 (150 - 180) |
| Operation time (min); median (IQR) | 174 (148 - 209) |
Clinical and Tumor Characteristics in Patients with PPGL a
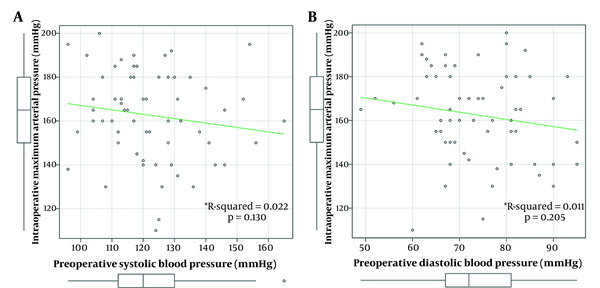
Relationship between preoperative blood pressure and intraoperative maximum arterial pressure
Intraoperative maximum AP showed a normal distribution (P = 0.618). Preoperative systolic BP and diastolic BP also showed normal distributions (P = 0.836 and P = 0.603, respectively). Neither preoperative systolic BP nor diastolic BP was correlated with intraoperative maximum AP (Figure 1A and B).
4.2. Normal Distributivity of Preoperative Continuous Variables
Among the continuous variables of preoperative clinical factors, age, and body mass index (BMI) showed normal distributions (P = 0.976 and 0.332, respectively). However, tumor size, the 24-h urinary-fractionated MN, and the 24-h urinary-fractionated NMN were not normally distributed (all P < 0.01). The logarithmic representation (log10) of these three continuous variables showed a normal distribution (P = 0.641, 0.201, and 0.715, respectively). Similarly, the 24-h urinary-fractionated MN and NMN was not normally distributed, but the logarithmic representation of this continuous variable also showed a normal distribution (P = 0.549).
4.3. Correlations Between Intraoperative Maximum APS and Preoperative Variables
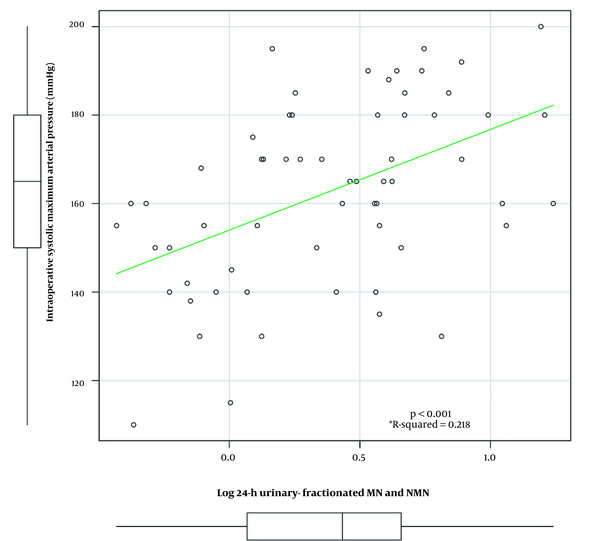
Log24-h urinary-fractionated MN and NMN was correlated with intraoperative maximum AP (R-squared = 0.218, P < 0.001; Figure 2). The correlation coefficient index was higher than each of log24-h urinary-fractionated MN and log24-h urinary-fractionated NMN (R-squared = 0.025 and R-squared = 0.156, respectively). Next, preoperative variables were investigated by linear regression analyses. Simple linear regression analyses showed that comorbidities of DM, one or more of the classic triad, and log24-h urinary-fractionated MN and NMN were significantly associated with intraoperative maximum AP (all P < 0.05; Table 2). Log24-h urinary-fractionated MN and NMN showed the highest t statistic among the preoperative variables (t statistic, 4.212). Then, multiple regression analyses showed that DM (β = 15.835, SE = 7.550, t statistic = 2.097, P = 0.040), one or more of the classic triad (β = 14.081, SE = 5.668, t statistic = 2.484, P = 0.016), and log24-h urinary-fractionated MN and NMN (β = 14.641, SE = 5.842, t statistic, 2.506, P = 0.015) were independent factors associated with intraoperative maximum AP (Table 2).
| Variables | Simple Linear Regression Analysis | Multiple Linear Regression Analysis a | ||||||
|---|---|---|---|---|---|---|---|---|
| β | SE | t Statistic | P Value | β | SE | t Statistic | P Value | |
| Age (y) | -0.111 | 0.173 | -0.643 | 0.522 | - | - | - | - |
| BMI (kg/m2) | 0.613 | 0.706 | 0.868 | 0.389 | - | - | - | - |
| Cerebro-cardiovascular events (absent vs. present) | -2.724 | 12.261 | -0.222 | 0.825 | - | - | - | - |
| Comorbidity of hypertension (absent vs. present) | 8.416 | 5.209 | 1.616 | 0.112 | - | - | - | - |
| Comorbidity of DM (absent vs. present) | 17.461 | 8.612 | 2.028 | 0.047 | 15.835 | 7.550 | 2.097 | 0.040 |
| One or more of the classic triad b (absent vs. present) | 19.788 | 5.452 | 3.629 | 0.015 | 14.081 | 5.668 | 2.484 | 0.016 |
| Tumor location (pheochromocytoma vs. paraganglioma) | -3.690 | 7.463 | -0.494 | 0.623 | ||||
| Type of surgery (open vs. laparoscopic) | -3.241 | 7.152 | -0.453 | 0.652 | - | - | - | - |
| Surgical approach (transperitoneal vs. retroperitoneal) | 8.854 | 8.242 | 1.074 | 0.287 | - | - | - | - |
| Log (tumor size, base = 10) | 4.955 | 12.115 | 0.409 | 0.684 | - | - | - | - |
| Log (24-h urinary-fractionated MN and NMN, base = 10) | 22.765 | 5.404 | 4.212 | < 0.001 | 14.641 | 5.842 | 2.506 | 0.015 |
| Preoperative systolic blood pressure (mmHg) | -0.202 | 0.175 | -1.155 | 0.253 | - | - | - | - |
Simple and Multiple Linear Regression Analyses to Assess the Association Between Intraoperative Maximum Arterial Pressure and Preoperative Variables in Patients with PPGL
5. Discussion
The present study found that either preoperative systolic or diastolic blood pressure was basically stable when adequate doses of α-receptor blockades had been administered, and neither was correlated with intraoperative maximum AP in patients with pheochromocytoma. We also demonstrated that log 24-h urinary-fractionated MN and NMN was correlated with intraoperative maximum AP. In addition, log 24-h urinary-fractionated MN and NMN, DM, and one or more of the classic triad were independent factors associated with intraoperative maximum AP. Thus, PPGL patients with these clinical factors might be at risk for hypertensive crises during surgery regardless of preoperative α-receptor blockades.
PPGL presents with various life-threatening complications, including cerebro-cardiovascular disease, HTN, and DM. Falhammar et al. showed that HTN and DM were present in 67% and 27% of patients with PPGL, respectively (12). Although our results showed that the incidence of these comorbidities were lower than reported (45.9% and 9.8%, respectively), in our cohort, tumor size was smaller than that of the cohort of Falhammar et al. (12) (median tumor sizes of 38 mm vs. 49 mm). These results indicated that patients in our cohort were diagnosed relatively early, and detecting the tumor while still small might reduce the prevalence of complications associated with PPGL.
PPGL also presents with a wide spectrum of symptoms related to excessive catecholamine secretion. The classic triad, which include palpitation, headache, and episode of sweating (the acronym PHE is reminiscent of pheochromocytoma), is valuable for screening symptoms for pheochromocytoma diagnosis. All three symptoms have specificities and sensitivities of more than 90% (13). However, recent retrospective reports have indicated that only 17% - 24% of pheochromocytoma cases exhibit all three symptoms (12, 14). In our cohort, the percentage of patients exhibiting all three symptoms of the classic triad was even lower; patients exhibiting at least one of the three symptoms accounted for only 26.2% of cases. This could be because the patients in our cohort were diagnosed with PPGL relatively early, as mentioned earlier. Taken together, the results indicate that the classic triad is unreliable for modern PPGL diagnosis. However, our study revealed that the presence at least one symptom of the classic triad was associated with intraoperative maximum AP. Consequently, the presence or absence of the classic triad should be confirmed to assess the preoperative severity of PPGL, even after it has been diagnosed.
In our study, intraoperative maximum AP was a primary outcome and assessed as a continuous variable; we confirmed that it was normally distributed. To examine the association of preoperative continuous variables with intraoperative maximum AP, it was necessary to validate their distributions. Factors that might be related to tumor growth, such as tumor size or 24-h urinary-fractionated metabolism of catecholamines, did not show normal distributions. However, the logarithmic representation of these continuous variables showed normal distributions. Interestingly, the logarithmic representation of tumor markers is important in assessing the progression or stage of particular types of cancer (15-17). Twenty-four-hour urinary-fractionated MN or NMN is used for biochemical screening but not disease progression or severity. Therefore, these values are considered important only for functional diagnosis of PPGL and evaluation of how many folds higher they are above the upper limit of normal (the cut-off value suggested by Japanese guidelines is three-fold). However, our results indicated that the 24-h urinary-fractionated metabolism of catecholamines should be evaluated as a logarithmic representation, similar to tumor markers; moreover, the logarithms of these variables were correlated with intraoperative maximum AP. Additionally, it was also indicated that evaluation of the sum of MN and NMN could reflect the severity of hormone secretion more accurately than either value alone.
Many experts, as well as Endocrine Society guidelines, continue to recommend preoperative α-receptor blockades to prevent hemodynamic instability and hypertensive crises for all patients with PPGL (5, 18). In an observational study, Groeben et al. reported that there was no difference in the incidence of excessive hypertensive crises between pheochromocytoma patients who had been administered α-receptor blockades and those who had not (6). They questioned whether all patients with pheochromocytoma require preoperative α-receptor blockades as prophylaxis. They also proposed that preoperative α-receptor blockades might not be mandatory for pheochromocytoma patients with low endocrine activity because of minimally invasive surgical techniques and highly effective drugs for intraoperative control of hemodynamic conditions. Unfortunately, we were not able to assess mild cases that might not require preoperative treatment. Meanwhile, our results revealed severe cases that were at risk for intraoperative hypertensive episodes regardless of preoperative α-adrenergic receptor blockers; therefore, patient selection is essential when considering precision preoperative management. We believe that PPGL patients can be divided into at least two categories: moderate and severe cases. Based on the opinion that there are some patients who would not require preoperative α-receptor blockades, mild cases can be added. Thus, there could be three categories. Adequate doses of preoperative α-receptor blockades would be expected to have an effect on moderate cases. However, preoperative α-receptor blockades alone might not be enough in severe cases, which have high levels of log 24-h urinary-fractionated MN and NMN, DM, or one or more of the classic triad. In these cases, additional preoperative interventions, such as metyrosine, which is catecholamine synthesis inhibitor, might help to stabilize intraoperative blood pressure.
Our results should be interpreted with caution because of several limitations. There were some ascertainment biases, which are inherent limitations of all retrospective observational analyses. Since the primary outcome was not perioperative hemodynamic instability, post-surgical hypotension and the use of catecholamines were not evaluated. Even though this study was not small compared with similar single-center studies, validation with a larger cohort would be required, mainly due to the rarity of PPGL. Also, we could not assess plasma free MN in most cases due to the system of Japanese insurance. Moreover, we were not able to decide the cut-off reference for log 24-h urinary-fractionated MN and NMN. To resolve these limitations, we will need to compare the accuracy of plasma free MN or urinary-fractionated MN for predicting intraoperative maximum AP in a larger cohort through a multi-center study.
5.1. Conclusions
Log24-h urinary-fractionated MN and NMN, DM, and one or more of the classic triad were independent factors associated with intraoperative maximum AP in patients with PPGL. Patients with these factors might be at risk for hypertensive crises during surgery regardless of preoperative α-receptor blockades. Clinicians should confirm these independent factors before surgery for PPGL and manage these patients more carefully and effectively.