1. Background
The intrauterine environment plays a vital role in offspring health (1, 2). Any hormonal, nutritional, and metabolic disorders in the intrauterine environment and during fetal development can predispose offspring to diseases in later life (3, 4). Prenatal exposure to environmental contaminants, such as lack of oxygen, pollutants, and nutritional factors, can disrupt the physiology and morphology of the reproductive system (5). In line with this hypothesis, many experimental studies have examined the role of environmental factors in epigenetic changes and reproductive health outcomes (6-8). For example, exposure to testosterone during the embryonic period has led to polycystic ovary syndrome (9, 10), Premature Ovarian Insufficiency (POI), and infertility in animals (11); these disorders may negatively affect only ovarian tissue or the HPO axis (12, 13).
The ovotoxicity effect of D-galactose, a monosaccharide in dairy products and fruits such as fig, has been previously reported (14-17). In addition, the accumulation of galactose metabolites in patients with galactosemia, a genetic disorder with an enzyme defect in galactose metabolism, leads to ovarian reserve deficiency (18). Based on emerging evidence, poor ovarian reserve is associated with estrogen deficiency and disorders such as infertility, cardiovascular disease, osteoporosis, and cognitional impairments (19-22).
Considering the lacked possibility of some investigations in humans due to ethical limitations, animals may be appropriate resources for studying the effects of D-galactose on the reproductive system. There is little data available on the impacts of prenatal D-galactose exposure on the reproductive health of female offspring (15, 23). In the study by Bandyopadhyay et al., the examination of ovaries in female offspring exposed to galactose during the fetal period was limited to the fetal period and 1-2 days after birth (23). It has been shown that in rodents, ovarian development is completed in early infancy, and primordial follicles are formed after 72 h of birth (24, 25); as a result, the assessment of ovarian follicles at 1-2 days after birth, as reported by Bandyopadhyay et al. (23), may not provide a precise estimate of ovarian reserves.
2. Objectives
In the present study, we aimed to examine the effect of prenatal D-galactose exposure on ovarian reserve in female rat offspring in later life (adolescence and adulthood).
3. Methods
3.1. Ethics Approval
The Ethics Committee of the Endocrine Sciences Research Institute of Shahid Beheshti University of Medical Sciences approved the present study (IR.SBMU.ENDOCRINE.REC.1398.001).
3.2. Design of Food Formulation
Food containing 35% D-galactose was prepared in the Research Institute for Endocrine Sciences laboratory. For one kilogram of D-galactose-enriched food, 350 g of D-galactose powder (Souvenir Chemicals, Mumbai, India) was added to 650 g of standard powdered food (Pars Co., Iran) and then prepared as dry pellets and stored in the refrigerator (2 - 4°C) for later use.
3.3. Animals and Care
Ten adult female Wistar rats (180 - 200 g) were obtained from the animal facility of the research institute. The rats were kept in a standard animal housing condition with a temperature of 23 ± 3°C, 50 - 55% humidity with proper ventilation, and 12-h light/dark cycles. Each female rat was mated with a healthy male rat for 24 hours in a separate cage. The presence of a vaginal plug was considered the first day of pregnancy.
3.4. Exposure to D-galactose
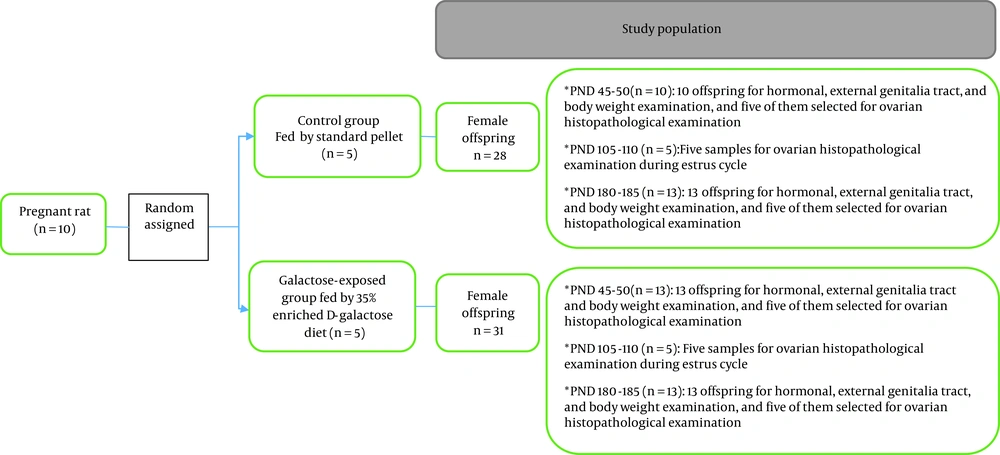
Ten pregnant rats were randomly divided into two groups. In one group, rats were fed with 35% D-galactose-enriched food from the third day of pregnancy to the end of pregnancy (n = 5), and in the other group, rats were fed a standard diet throughout their pregnancy (n = 5). Female offspring of both groups (prenatally galactose-exposed rats and non-exposed control rats) were examined in terms of the ovarian index (ovarian weight/body weight), ovarian volume, and ovarian histology (number of primordial and atretic follicles) at three different ages (40 - 45, 105 - 110, and 180 - 185 days as postnatal days (PND)), when they were in the estrus phase of their sexual cycles. The serum levels of anti-mullerian hormone (AMH), follicle-stimulating hormone (FSH), and estradiol (E2) were measured at PND of 45 - 50 and 180 - 185. External genitalia tract, time of vaginal opening, and the regularity of estrous cycle were examined in all study rats. Moreover, body weights were measured at various ages. A summary of the study protocol is presented in Figure 1.
3.5. Puberty, External Genital Tract Examination, and Estrous Cycle
The vaginal opening as an indicator of the puberty onset was checked in all study rats (prenatally galactose-exposed rats and controls) at 30 - 45 days of age. The day of vaginal opening was recorded for each rat separately. The characteristics of the external genitalia, including the length of the vagina and clitoris, anogenital distance (AGD; the distance (in millimeters) between the cranial edge of the anus and the base of the phallus), and anovaginal distance (AVD; the distance (in millimeters) between the anterior edge of the anus and the posterior edge of the vaginal orifice) were measured using a vernier caliper in adulthood (100 - 105 days of age). After puberty, the regularity of the estrous cycle was examined for 10 consecutive days (65 - 75 days of age). To determine different estrous cycle phases, we prepared vaginal smears daily. The collection and staining of vaginal fluid samples were explained in a previous study (26).
3.6. Determination of Body Weight
The body weights of prenatally galactose-exposed rats and controls were measured at birth and every 15 days until 120 days of age, and then once a month.
3.7. Blood Collection and Serum Sampling
During 45 - 50 and 180 - 185 days of age, when prenatally galactose-exposed rats and their controls (n = 10 - 13 in each group) were at the estrus phase of the sexual cycle, blood samples were collected from the abdominal aorta after inducing deep anesthesia using i.p. injection of pentobarbital sodium (Sigma Aldrich, P3761-5 g; 60 mg/kg body weight). Blood samples were centrifuged at 10,000 rpm for 5 min at 4°C. The sera were placed at -80°C for subsequent measurement of FSH, E2, and AMH hormone levels. Hormonal measurements were performed using ELISA kits based on the manufacturer's protocol [Rat FSH ELISA Kit (Cat. No.: ZB-10182C-R9648, sensitivity 0.12 mIU/mL; Zellbio, Germany), E2 ELISA Kit (Cat. No.: 4925-300A; Monobind, USA, sensitivity 8.2 pg/mL); Rat AMH ELISA Kit (Lot ZB-MI19822; sensitivity 0.05 ng/mL, Zellbio, Germany)]. Intra-assay coefficients of variation for all hormones were < 10%.
3.8. Evaluation of Ovaries
3.8.1. Ovarian Tissue Extraction and Histological Evaluation
After blood collection, the right ovaries of prenatally galactose-exposed rats and controls were removed, quickly trimmed, weighed, and fixed in formalin (10%) for seven days at room temperature. Systematic uniform random sampling was used to quantify the ovarian follicles. For this purpose, the ovaries were embedded in paraffin, and serial sections with 10 μm thicknesses were prepared based on a standard tissue preparation method. For each sample, the first section containing ovarian tissue was chosen, and the following sections were selected by intervals revealed by dividing all sections into 10. All 10 selected sections from each ovary per offspring were mounted and stained using hematoxylin and eosin (H&E) (Merck, Darmstadt, Germany). The counting of primordial and primary follicles throughout the whole ovary is necessary to provide an accurate number since these follicles are not distributed equally throughout the ovaries; however, it has been shown that even five randomly selected sections are sufficient to precisely assess the impact of various ovotoxic agents on ovarian follicles and estimate their fertility (27).
Ovarian tissue sections were examined using a light microscope at 40X magnification (Nikon's DS-Fi1, Japan). The primordial, primary, and atretic follicles were counted separately in each section in a spiral manner using the microscope stage along the X and Y axes. The tissue section and sampling were performed by one person (MN), wholly blinded to the study groups. A person (MRD) blinded to the study groups counted the follicles. Each sample was recorded by a number obtained from the random number table. The follicles were categorized based on a method described by Myers et al. (28) as follows: Primordial follicle: a follicle containing an oocyte with a row of flat follicular cells around it; primary follicle: follicle containing an oocyte with a row of cuboidal cells around it (if more than three cuboidal follicular cells were observed around the oocyte, it was considered the late primary follicle); and atretic follicles: follicle with shrinkage of the oocyte and irregular shape.
3.8.2. Determination of Ovarian Volume
Cavalieri's method determined the ovarian volume (29). Appropriate sections were selected by a systematic random sampling method, and the total points that hit the region of interest were calculated using the Cavalieri/point-counting estimator (point grid with appropriate spaces). The total volume of the ovary (mm3) was calculated using the following equation (30):
In this formula, ΣP is the total number of points hitting the tissue section, T is the distance between the tissue sections, and
3.9. Statistical Analysis
The generalized estimation equations (GEE) method was applied to investigate secular longitudinal trends of body weight in both groups (galactose-exposed and control). It accounts for correlations within subjects through a working correlation matrix. The interaction between the group status (prenatally galactose-exposed rats vs. controls) and study time points was tested. An exchangeable working correlation matrix that accounts for correlations within subjects was implemented. Predictors were age (days), group status, and their interaction (age (days) × group status). All data were presented as mean ± SEM. Also, P-value < 0.05 in the independent sample t-test was considered significant. Statistical analyses were performed using SPSS (version 20) and the software package STATA (version 13; STATA Inc., College Station, TX, USA).
4. Results
4.1. Onset of Puberty, External Genital Tract, and Estrous Cycle
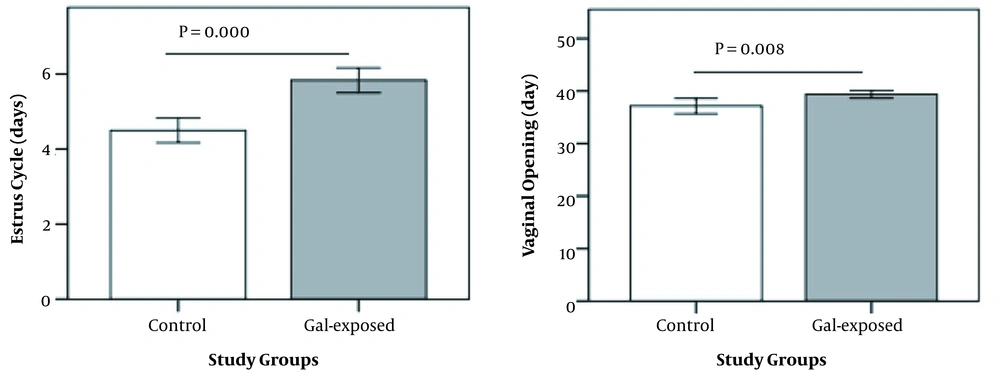
Delay in puberty was observed in prenatally galactose-exposed rats compared to controls (39.4 vs. 37.1 days of age; P = 0.000). No significant differences were observed in AGD, AVD, and the lengths of the vagina and clitoris between the two groups (data not shown). Prolonged estrous cycles were observed in prenatally galactose-exposed rats compared to controls (5.8 vs. 4.5 days; P = 0.002) (Figure 2).
4.2. Body Weights
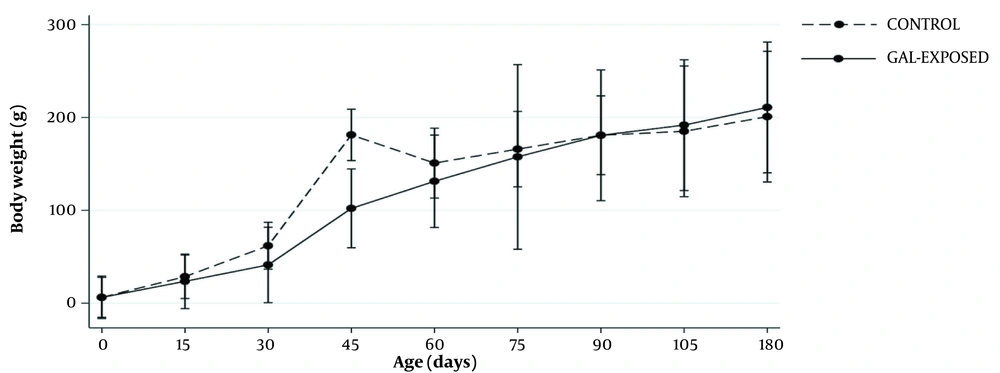
Trends in body weights (BWs) during the follow-up based on GEE analysis are shown in Figure 3. The mean changes in BWs had no statistically significant differences between galactose-exposed rats and controls over time (mean difference: -3.04, 95% CI: -11.38 - 5.29, P interaction = 0.5).
The mean of body weight changes in follow-ups in prenatally Gal-exposed rats and controls using generalized estimating equation (GEE), assuming the interaction between time and study group. Gal-exposed: Female offspring exposed to D-galactose during their prenatal life. Gal-exposed: Female offspring exposed to D-galactose during their prenatal lives. Bars indicate standard errors.
4.3. Hormonal Levels
The serum levels of AMH, FSH, and E2 were compared between prenatally galactose-exposed rats and their controls at PND of 45 - 50 and 180 - 185. The results distinguished a significant reduction in AMH and E2 hormonal levels at PND of 45 - 50 and a significant increase in FSH levels at the same PND, but not at PND of 180 - 185, in galactose-exposed rats compared to controls (Table 1).
| Groups of Study | AMH (ng/mL) | P-Value | E2 (pg/mL) | P-Value | FSH (mIU/mL) | P-Value |
|---|---|---|---|---|---|---|
| PND 45 - 50 | 0.000 | 0.002 | 0.01 | |||
| Control (n = 10) | 7.6 ± 0.12 | 21.7 ± 3.22 | 4.03 ± 0.42 | |||
| Galactose-exposed (n = 13) | 3.3 ± 0.53 | 7.7 ± 1.3 | 8 ± 1.3 | |||
| PND 180 - 185 | 0.08 | 0.4 | 0.9 | |||
| Control (n = 13) | 5.52 ± 0.12 | 19.5 ± 4.01 | 3.23 ± 0.52 | |||
| Galactose-exposed (n = 13) | 4.2 ± 0.70 | 14.9 ± 2.61 | 3.22 ± 0.2 |
Comparison of AMH, E2, and FSH Levels Between Study Groups a
4.4. Ovarian Characteristics and Histopathologic Changes
4.4.1. Macroscopic Changes
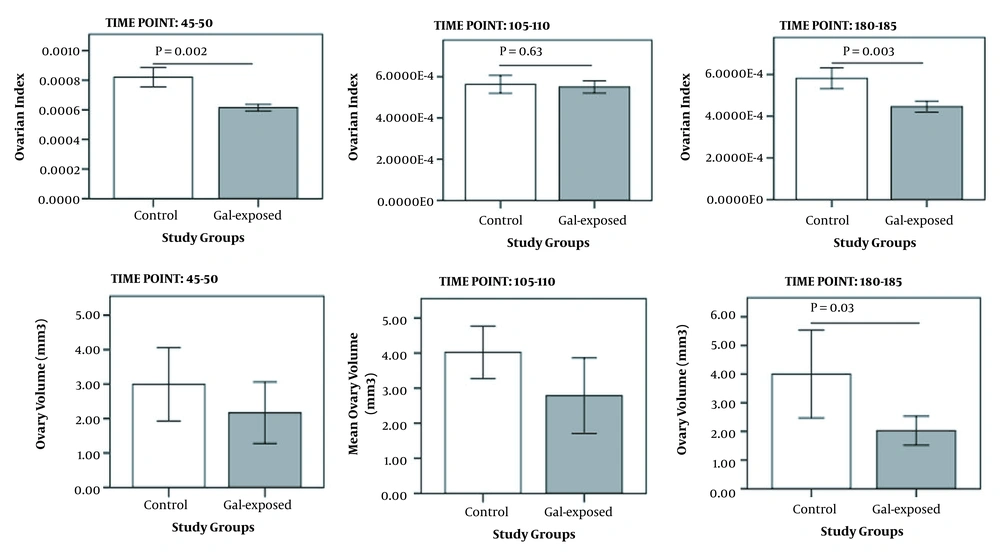
Ovarian index significantly decreased in galactose-exposed rats compared to controls. This reduction was significant at PND of 45 - 50 (P = 0.004) and 180 - 185 (P = 0.02); however, the ovarian volume was significantly different between the two groups only at PND of 180 - 185 (P = 0.03) (Figure 4).
4.4.2. Microscopic Changes: Ovarian Follicles Examination
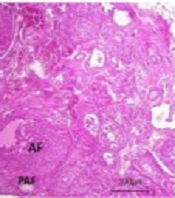
The primordial, primary, and atretic follicles were counted thrice (45 - 50, 105 - 110, and 180 - 185 days of age) to compare the ovarian reserve and the irreversibility of changes between galactose-exposed rats and controls (Figure 5).
Histological examination of ovarian tissue [hematoxylin and eosin (H&E, 40X magnification)]; A and B: Ovarian sections in prenatally gal-exposed rats and controls, respectively (45 - 50 days of age); C and D: Ovarian sections in prenatally gal-exposed rats and controls, respectively (105 - 110 days of age); E and F: Ovarian sections in prenatally gal-exposed rats and controls, respectively (180 - 185 days of age). PF, primary follicle; PMF, primordial follicle; AF, antral follicle; PAF, preantral follicle; ATF, atretic follicle; CL, corpus luteum.
Significant reductions in primordial follicle numbers were detected in prenatally galactose-exposed rats compared to the controls in a timely manner (PND 45 - 50: P = 0.002; PND 105 - 110: P = 0.002; PND 180 - 185: P = 0.008). The primary follicle numbers significantly increased (P = 0.04) at PND of 105 - 110 and then decreased (P = 0.03) at PND of 180 - 185 in prenatally galactose-exposed rats. At PND of 45 - 50, a significant increase in the number of atretic follicles was observed in prenatally galactose-exposed rats compared to controls (86.1 vs. 23, respectively, P = 0.01) (Table 2). The differences in the numbers of corpus luteum were statistically significant at PND of 45 - 50 and 105 - 110 (P = 0.04 and P = 0.01, respectively) but not at PND of 180 - 185 (P = 0.06).
| Study Groups | Primordial Follicle Number | P-Value | Primary Follicle Number | P-Value | Corpus Luteum Number | P-Value | Atretic Follicle Number | P-Value |
|---|---|---|---|---|---|---|---|---|
| PND 45 - 50 | 0.002 c | 0.12 | 0.04 b | 0.002 c | ||||
| Control (n = 5) | 62.2 ± 8 | 51 ± 5.3 | 54.40 ± 5.42 | 23 ± 11.6 | ||||
| Galactose-exposed (n = 5) | 30.3 ± 2.26 | 39.1 ± 4.4 | 40.0 ± 3.34 | 86.1 ± 9.9 | ||||
| PND 105 - 110 | 0.002 c | 0.06 | 0.01 b | 0.01 b | ||||
| Control (n = 5) | 33 ± 1 | 40 ± 1.21 | 66.80 ± 7.01 | 33 ± 4.61 | ||||
| Galactose-exposed (n = 5) | 23.8 ± 1.6 | 32.4 ± 0.7 | 37.40 ± 6.0 | 61 ± 8.37 | ||||
| PND 180 - 185 | 0.000 d | 0.03 b | 0.06 | 0.2 | ||||
| Control (n = 5) | 28.4 ± 2.5 | 39 ± 4.4 | 46 ± 5.0 | 28.4 ± 9.13 | ||||
| Galactose-exposed (n = 5) | 11.4 ± 0.5 | 24.2 ± 4 | 32.40 ± 4.0 | 47 ± 11.4 |
Comparison of Different Growing Follicle Stages and Atretic Follicles Between Groups a
5. Discussion
In the present study, the gradual reduction in primordial ovarian follicles over time was more prominent in prenatally galactose-exposed rats. In addition, the endocrinological features of ovaries in terms of hormonal changes were affected by D-galactose exposure during fetal life. Moreover, increased FSH levels in prenatally galactose-exposed rats compared to controls indicated no adverse effect on the hypothalamus-pituitary-ovary (HPO) axis in adulthood. The lack of a significant weight difference between the study groups during the follow-up eliminates the possibility of an indirect effect of D-galactose feeding on the current observations.
There is ample evidence of an increase in the risk of developing adult diseases due to non-genomic changes during the fetal period (31). The early growth of various body organs, including the reproductive system, may be impaired by exposure to toxins, nutrient intake, diet composition, air pollutants, or maternal metabolic disorders (32). The healthiness of embryonic life is essential for developing normal ovaries containing high-quality follicles and an efficient reproductive system in females in adulthood (33). The relationship between fetal nutrition and mammalian ovarian health in adulthood seems logical, as oogenesis onsets in the early embryonic period (34, 35).
The ovarian follicle formation in rats, as the functional unit of ovaries, begins in the embryonic period with the migration of primordial germ cells (PGCs) from the yolk sac to the gonadal ridge from the third day of conception (24). During the first two weeks of pregnancy, the migration of PGCs can be influenced by external factors (36). Exposure to a D-galactose-enriched diet during the embryonic period disrupts the expression of growth differentiation factor-9 (GDF-9) and impairs PGCs migration (36). In addition, the expression of N-acetylgalactosamine (GalNAc) has been shown transiently and selectively at the surface of PGCs during their migration in rats (37). It is suggested that GalNAc at PGCs surfaces may play a functional role in regulating the conduction and movement of these cells during their extensive migration. The embryonic time for GalNAc expression between the eighth and 15th days of gestational age and the 12th day of pregnancy in rats is critical for sexual and neuroendocrine differentiation mediated by gonadotropins (38-41). As observed in the present study, the number of primordial follicles in prenatally galactose-exposed rats was significantly decreased and continued up to adulthood (PND of 180 - 185).
Serum AMH is a good indicator of ovarian reserve (41, 42). This hormone is explicitly expressed in granulosa cells of growing follicles from the primary to antral follicles and is regulated by FSH (43). The primary function of granulosa cells is to produce sex steroids. In the present study, the AMH level at PND of 45 - 50 was significantly decreased in the galactose-exposed offspring group, reflecting the ovarian reserve (P = 0.000). The gradual reduction of primordial follicle numbers and decreased AMH and E2 levels in galactose-exposed offspring at PND of 45 - 50 indicate the adverse effect of D-galactose on ovarian reserve and its hormonal production.
The secondary sexual characteristics emerge with puberty. The onset of the estrous cycle is a secondary sexual characteristic that begins after puberty. The coordination of the HPO axis regulates the estrous cycle. Impaired ovarian function under D-galactose toxicity may delay puberty; however, dysfunction of upstream centers under D-galactose toxicity is unclear. Rats' nervous system and ovaries are not yet fully differentiated at birth (44). It means that the possible impact of prolonged exposure to ovotoxic agents during the fetal period influences advanced neuroendocrine system function and HPO axis feedback, which may present options for hormonal disruption. In this study, the possible interference with the HPO axis may be confirmed by irregular estrus cycles and delayed puberty in galactose-exposed offspring, similar to those disturbances in women's menstrual cycles with a poor ovarian reserve in POI (45-47). However, increased FSH levels following E2 reduction in physiological feedback, as observed in our study, reject the HPO axis disturbance. Despite these contradictory findings, the effect of D-galactose on the upstream centers of the HPO axis is unknown and needs further investigation. Taking more than 6 g of D-galactose daily in women increases the FSH hormone level (16); however, there is no evidence of a relationship between D-galactose consumption and the occurrence of premature menopause in women (48).
5.1. Strengths and Limitations of the Study
Since most investigations of D-galactose exposure have been performed during the postnatal period, the examinations of prenatal D-galactose exposure and long-lasting effects on the ovarian reserve are the strengths of the current study. However, the present study has several limitations. We did not investigate the toxicity of D-galactose on other organs, e.g., the brain. The present study did not assess immunological and aging parameters, including anti-ovarian antibodies and inflammatory factors such as interleukins. It would be better to examine the dam's weight gain during pregnancy to fully clarify the hypothesis of receiving extra energy through galactose consumption. However, the lack of difference in the offspring's weight between galactose exposed and non-exposed groups may indirectly eliminate the significant effect of dieting with D-galactose on extra energy intake of dams during their pregnancy period. The similarity in body weight may not exclude the difference in body composition between the study groups; as a result, a lack of body composition assessment may be a limitation to the present study. While the similarity of offspring's weight between the exposed and non-exposed groups may show a similar energy intake of dams during their pregnancy periods, the lack of weight gain assessment can be another limitation to the present study. Further comprehensive studies can determine the effect of D-galactose on other organs and assess hormonal, cytological, and immunological changes in ovaries at various time points and the fertility potential of prenatally D-galactose-exposed rats.
5.2. Conclusions
Prenatal exposure to D-galactose negatively affects ovarian reserve in female rats in their later lives. However, further investigation is needed to confirm these findings and explore underlying mechanisms.
![Histological examination of ovarian tissue [hematoxylin and eosin (H&E, 40X magnification)]; A and B: Ovarian sections in prenatally gal-exposed rats and controls, respectively (45 - 50 days of age); C and D: Ovarian sections in prenatally gal-exposed rats and controls, respectively (105 - 110 days of age); E and F: Ovarian sections in prenatally gal-exposed rats and controls, respectively (180 - 185 days of age). PF, primary follicle; PMF, primordial follicle; AF, antral follicle; PAF, preantral follicle; ATF, atretic follicle; CL, corpus luteum. Histological examination of ovarian tissue [hematoxylin and eosin (H&E, 40X magnification)]; A and B: Ovarian sections in prenatally gal-exposed rats and controls, respectively (45 - 50 days of age); C and D: Ovarian sections in prenatally gal-exposed rats and controls, respectively (105 - 110 days of age); E and F: Ovarian sections in prenatally gal-exposed rats and controls, respectively (180 - 185 days of age). PF, primary follicle; PMF, primordial follicle; AF, antral follicle; PAF, preantral follicle; ATF, atretic follicle; CL, corpus luteum.](https://services.brieflands.com/cdn/serve/3170b/f7132500e63fa36c1ce9884bb784f44c08080977/ijem-123206-g001-F5-preview.webp)