1. Background
The International Diabetes Federation (IDF) has estimated that up to 80% of type 2 diabetes mellitus (T2DM) cases occur in low- and middle-income countries (1). In absolute numbers, the proportion of patients with T2DM in low- and middle-income countries is growing much faster than that in high-income countries (2). Despite the availability of cost-effective medications with proven efficacy (3), health care systems in low- and middle-income countries have been continually challenged by the growing need for care for patients with T2DM (4). Scaling up the provision of care for patients with T2DM in resource-limited settings is presently a major challenge in low- and middle-income countries.
Leading medical organizations, such as the American Diabetes Association (ADA), have recommend that all patients over 45 should be screened for prediabetes and T2DM and that patients with risk factors should be screened even earlier (5-7). The early identification of patients with T2DM or its risk factors facilitates better economic planning and utilizing healthcare resources. However, the organization of such a screening program with limited resources is challenging (8).
According to the World Bank, the Republic of Uzbekistan is a lower-middle-income country (9), and according to IDF estimates, the prevalence of diabetes mellitus (DM) in Uzbekistan is 5.4% (10); however, some studies have reported an estimated prevalence of up to 9.1% for the country (11). The frequency of diagnosed T2DM in Uzbekistan is 650 cases per 100 000 (12), which is lower than the percentage of diagnosed patients in neighboring countries (13).
Moreover, screening studies have revealed an increase in the prevalence of T2DM among those aged 35 and older in the Republic of Uzbekistan in recent years (14). In a recalculation for a population of 30 million, the total number of patients with T2DM has been assumed to be approximately 1.2 million, which does not correspond with the number of officially registered cases (approximately 231,000). Thus, for every patient with an established diagnosis of T2DM, there are approximately 5 - 6 patients without an established diagnosis. In addition, according to the annual reports of regional dispensaries, approximately 80% of patients with T2DM in the Republic of Uzbekistan do not reach the target values of glycemia (12). It is known that inadequate glycemic control contributes to the development of complications that lead to reduced life expectancy (15, 16), which is also observed in the Republic of Uzbekistan (17); thus, the issue of high risk of development and progression of cardiovascular complications and chronic microvascular diabetic complications is acute.
Several variants of T2DM screening have been proposed, but the applicability of these approaches to the condition of the Republic of Uzbekistan is questionable; for example, the FINDRISK questionnaire, the most popular and well-proven tool, has not been validated for the Central Asian population. According to the studies using the FINDRISK questionnaire in the Republic of Uzbekistan, up to 60% of patients with dysglycemia have a low or moderate risk (18). In this regard, the issue of regular cost-effective screening of T2DM in the Republic of Uzbekistan is acute.
2. Objectives
The present study, taking into account the limited resources of Uzbekistan's health care system, aimed to evaluate the nationwide screening program in order to find the most effective, simple, and economic option required for the actual implementation of regular T2DM screening in the country’s primary care.
3. Methods
DM screening was conducted from December 2018 to March 2019 in family polyclinics in the urban and rural areas of two regions of Uzbekistan after obtaining the approval from the Ministry of Health of the Republic of Uzbekistan and relevant ethics committees. All participants signed an informed consent form voluntary before participation in the study.
The pilot was conducted in a factorial design format with cluster randomization of participants across the sites included in the pilot. Data from all patients meeting the eligibility criteria and referring to the general practitioners (GP) for any reasons were included for analysis. The patients informed about the ongoing screening through the advertisements posted in GP offices were enrolled in the study.
Patients were randomized 2 times:
- Depending on the number of eligibility criteria: 1 (≥ 45 years) or 3 eligibility criteria (≥ 45 years of age & BP ≥ 140/80 mmHg OR taking antihypertensive medications & obesity (BMI ≥ 30 kg/m2, waist circumference (WC) ≥ 80 cm in women and ≥ 90 cm in men))
- Depending on how the diagnosis of T2DM was confirmed (fasting glucose the day after a random glycemic determination or glycated hemoglobin on the same day as the first screening glycemic determination).
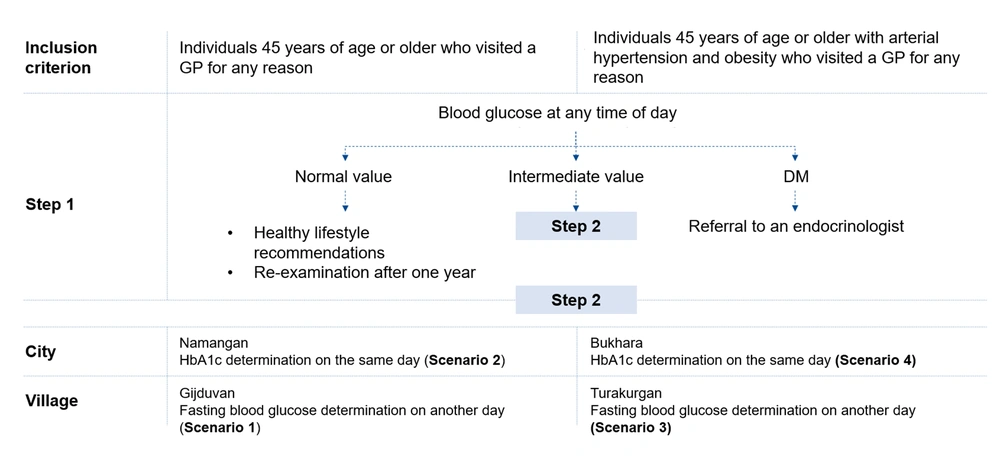
Thus, there were four different scenarios (Figure 1), which differed in terms of eligibility criteria and the methodology adopted for detecting dysglycemia. In some of the clusters, patients were allowed to take part in the screening if they met the 1 inclusion criterion (≥ 45 years). In another series of clusters, if they had to meet the three inclusion criteria. Patients independently decided whether the inclusion criteria met and whether to undergo screening. Pre-specified data analysis intention-to-treat (ITT) assumed that the main analysis of the dataset would be performed in a full sample of patients, regardless of whether they correctly identified themselves with the group suitable for screening or not (e.g., whether they would correctly calculate their BMI). The data on a group of patients meeting all inclusion criteria per-protocol (PP) (Appendix 1) were additionally analyzed.
In step one, one screening criterion was used in Gijduvan and Namangan, and three screening criteria were used in Bukhara and Turakurgan. The screening was performed by pretrained medical personnel: General practitioners (GPs) and district nurses. Prior to conducting the pilot, the training was carried out once in the form of a 2-hour workshop. In the case of hyperglycemia detection, patients were invited for the second visit (step 2) with regard to the scenario (Figure 1).
Glycemia was determined by test strips using a OneTouch glucometer in capillary blood plasma:
- Fasting glycemia: < 6.1 mmol/L - normal (recommendations given); ≥ 7.1 mmol/L - referral to an endocrinologist; intermediate value - repeated checkup for confirmation (step 2).
- Incidental glycemia: < 7.8 mmol/L - normal (recommendations given); ≥ 11.1 mmol/L - referral to an endocrinologist; intermediate value - repeated checkup for confirmation (step 2).
Glycated hemoglobin (HbA1c) was determined using Siemens reagents:
< 6.5% - normal; ≥ 6.5% - referral to an endocrinologist.
The primary endpoint of the study was the proportion of T2DM diagnoses confirmed by an endocrinologist. The secondary endpoints were the proportion of diabetes cases detected by the GPs, cases of prediabetes detected by the GPs, and the cost of consumables needed to detect one case of diabetes mellitus. In addition, an analysis of the T2DM incidence depending on sex, age, blood pressure, and BMI was performed.
Despite the given instructions, the GPs also surveyed individuals aged under 45 years, which revealed the degree of adherence to the instructions in actual clinical practice. The data obtained after the examination of individuals aged under 45 years were used to further analyze the rationale for the age threshold for inclusion in the study.
Statistical analysis was performed using Microsoft Excel and SPSS 26.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics (i.e., frequency of distribution, absolute values, estimated distribution, mean values, etc.) were used to obtain general characteristics of the results distribution, as well as to compare different groups regarding the data distribution. Nonparametric comparison criteria for two or more independent and dependent samples (i.e., Mann-Whitney U test, Kolmogorov-Smirnov test, Wald-Wolfowitz runs test, Wilcoxon test, chi-square test, and binomial test) were used to evaluate differences between parametric and nonparametric data groups. Parametric comparative t-tests for two or more independent and dependent samples were performed to assess differences between two groups of normally distributed quantitative parameters.
4. Results
A total of 2,420 patients were examined during four months: 592 patients in Bukhara, 601 patients in Gijduvan, 604 patients in Namangan, and 624 patients in Turakurgan. A total of 1,741 (71.6%) of the patients were females, and 689 (28.4%) of them were males. Out of the examined individuals, 22% (21% of the females and 24% of the males) had a BMI within normal limits, 40% (37% of the females and 48% of the males) were overweight (BMI 25.0 - 29.9 kg/m2), and 38% (42% of the females and 28% of the males) were obese (BMI ≥ 30.0 kg/m2).
Tables 1 and 2 show the baseline characteristics of the patients included in the study according to different numbers of eligibility criteria and depending on the method of diagnosis confirmation as well as the glycemia assessment results. Generally, there were no significant differences between ITT and PP populations. Here, the data of the ITT population are presented.
| 1 Eligibility Criterion | 3 Eligibility Criteria | P | |
|---|---|---|---|
| Total (N) | 1205 | 1215 | |
| Males (%) | 29.6 | 27.3 | 0.21 |
| Females (%) | 70.4 | 72.7 | 0.21 |
| Age (y) | 56 ± 12 | 56.5 ± 9 | 0.289 |
| SBP (mmHg) | 126.7 ± 19.7 | 131.3 ± 19.1 | < 0.001 |
| DBP (mmHg) | 82.6 ± 12.6 | 83.2 ± 24.8 | 0.392 |
| Height (cm) | 161.4 ± 7.9 | 161.8 ± 8.6 | 0.151 |
| Weight (kg) | 75.8 ± 14.7 | 79.2 ± 15.9 | < 0.001 |
| BMI (kg/m2) | 29.1 ± 5.6 | 30.3 ± 5.8 | < 0.001 |
| WC (cm) | 96.5 ± 12.8 | 100.1 ± 15 | < 0.001 |
| Proportion of obese patients | 38.0 | 47.4 | < 0.001 |
| HbA1c | Glucose | ||
| Total (N) | 1195 | 1225 | |
| Males (%) | 27.8 | 29.1 | 0.45 |
| Females (%) | 72.2 | 70.9 | 0.45 |
| Age (y) | 56.1 ± 9 | 56.4 ± 12 | 0.538 |
| SBP (mmHg) | 125.5 ± 17.8 | 132.5 ± 20.5 | < 0.001 |
| DBP (mmHg) | 81 ± 25.6 | 84.8 ± 11 | < 0.001 |
| Height (cm) | 161.9 ± 8.8 | 161.3 ± 7.7 | 0.092 |
| Weight (kg) | 79 ± 15.4 | 76.1 ± 15.2 | < 0.001 |
| BMI (kg/m2) | 30.2 ± 6 | 29.2 ± 5.3 | < 0.001 |
| WC (cm) | 98.2 ± 14.5 | 98 ± 13.5 | 0.758 |
a Data are presented as mean ± SD.
| 1 Eligibility Criterion | 3 Eligibility Criteria | P | |
|---|---|---|---|
| Incidental glycemia (mmol/L) | 7.0 ± 2.9 | 7.8 ± 3.6 | < 0.001 |
| Fasting glycemia (mmol/L) | 6.5 ± 2.6 | 6.8 ± 2.5 | 0.047 |
| HbA1c (%) | 7.5 ± 2.9 | 6.4 ± 1.6 | < 0.001 |
| Proportion of obese patients | 38.0 | 47.4 | < 0.001 |
| HbA1c | Glucose | ||
| Incidental glycemia (mmol/L) | 6.9 ± 3.1 | 7.8 ± 3.5 | < 0.001 |
| Fasting glycemia (mmol/L) | 6.6 ± 2.6 | 6.8 ± 2.5 | 0.300 |
| HbA1c (%) | 6.8 ± 2.3 | - | < 0.001 |
a Data are presented as mean ± SD.
The diagnosis of T2DM was established by an endocrinologist in 9.3% of the cases with one eligibility criterion and 15.9% of the cases with three eligibility criteria (P = 0.000001, Table 3). The diagnosis of T2DM was established by an endocrinologist in 11.7% of the cases with HbA1c screening and 13.5% of the cases with glucose screening (P = 0.19, Table 3).
| 1 Eligibility Criterion (%) | 3 Eligibility Criteria (%) | P | |
|---|---|---|---|
| T2D (endocrinologist) | 9.3 | 15.9 | 0.000001 |
| T2D (GP) | 9.4 | 15.3 | 0.000010 |
| Prediabetes (GP) | 11.7 | 20.6 | 0.000001 |
| HbA1c | Glucose | ||
| T2D (endocrinologist) | 11.7 | 13.5 | 0.191 |
| T2D (GP) | 13.5 | 11.2 | 0.077 |
| Prediabetes (GP) | 11.3 | 20.9 | 0.000001 |
Abbreviations: T2D, type 2 diabetes; GP, general practitioner.
T2DM detectability by primary care physicians in sites with three eligibility criteria were 13.5% and 18.1% in Bukhara and Turakurgan (P = 0.028), respectively; and that in sites with one eligibility criterion were 8.7% and 9.9% in Gijduvan and Namangan (P = 0.44), respectively. All cases of discrepancies in the result assessment were analyzed by the project coordinator working with primary care physicians. In addition, the GPs detected 20% of prediabetes cases in Bukhara, Gijduvan, and Turakurgan, while it detected 9% of prediabetes cases in Namangan, which was 17% in total.
The detectability was discovered to increase with age among female participants. In contrast, the detectability peaked for male participants aged 50 - 59 years and then decreased (Table 4). With a normal BMI and one screening criterion, T2DM was diagnosed in only 4.6% of the patients, whereas the adoption of three criteria facilitated the diagnosis in 9.9% of the patients. A similar trend was observed among overweight (i.e., 10.1% for one criterion and 16.4% for three criteria) and among obese patients (i.e., 11.1% for one criterion and 17.4% for three criteria). Overall, 6.8% of the patients with a BMI within normal limits, 13.1% of overweight patients, and 14.6% of obese patients were diagnosed with T2DM. The prevalence of T2DM increased when BMI increased.
| Age (y) | |||||||
|---|---|---|---|---|---|---|---|
| Under 40 | 40 - 44 | 45 - 49 | 50 - 59 | 60 - 69 | 70 - 79 | Over 80 | |
| Males (%) | 12.5 | 19 | 12.9 | 18.1 | 13.6 | 14.9 | 0 |
| 95% CI | 1.4 - 45.4 | 6.8 - 39.2 | 7.4 - 20.4 | 13.7 - 23.2 | 9.8 - 18.3 | 6.9 - 27 | 0 - 0 |
| Females (%) | 4.5 | 8.2 | 5.9 | 11.0 | 16.5 | 18.1 | 22.2 |
| 95% CI | 0.5 - 19.3 | 3.9 - 14.8 | 3.7 - 8.7 | 8.9 - 13.4 | 13.3 - 20.1 | 10.5 - 28.1 | 4.9 - 54.4 |
When one criterion was used, males with systolic blood pressure (SBP) < 140 mmHg were found to have T2DM in 11.2% of cases, whereas males with an SBP ≥ 140 mmHg were discovered to have T2DM in 14.2% of cases. As for female patients, these rates were 5.6% and 14.8%, respectively. A total of 7.1% of patients with an SBP < 140 mmHg and 14.6% of patients with an SBP ≥ 140 mmHg were found to have T2DM in scenarios with one eligibility criterion.
Adopting three eligibility criteria allowed a diagnosis of T2DM in 15.8% of the males with an SBP < 140 mmHg and 21.3% of the males with an SBP ≥ 140 mmHg. These rates for females were 15.5% and 14.1%, respectively. A total of 15.6% of the patients with an SBP < 140 mmHg and 16.3% of the patients with an SBP ≥ 140 mmHg were diagnosed with T2DM.
4.1. Cost of Identifying one Patient with T2DM
The cost of identifying one patient with T2DM was determined according to the method of screening and the number of eligibility criteria. In the scenario with HbA1c determination, the cost of identifying one patient with T2DM was 83,000 soms, whereas the cost of identifying one patient with recurrent glycemia determination was 21,500 soms. Thus, the difference in cost was 286% (Appendix 2). As for the time when one risk factor was considered, the cost of identifying one patient with T2DM was 59,397 soms; and as for the time when three risk factors were taken into account, the cost was 44,119 soms. The difference in cost was 25.7%.
The HbA1c level at the time of diagnosis in 42% of the patients with newly diagnosed T2DM ranged between 6.5 and 7.0%, it ranged between 7.0 and 8.9% in 25% of the patients, and it exceeded 9.0% in 33% of the patients.
A total of 8.0% of the patients with newly diagnosed T2DM had fasting glucose levels below 7.0 mmol/L (i.e., the diagnosis was made by postprandial glycemia and/or HbA1c), 51.6% had fasting glucose levels of 7.0 - 11.1 mmol/L, and 40.4% had fasting glucose levels above 11.0 mmol/L.
Adoption of screening for three risk factors facilitated the detection of prediabetes (IGT and IFG) in 19.8% of the patients, while prediabetes was detected in 14.4% of cases in the group of patients older than 45 years without additional risk factors. The adoption of HbA1c as an additional diagnostic method facilitated the detection of 14.1% of prediabetes cases, and the recurrent glycemic determination facilitated detecting 20.2% of the cases.
Analyzing the sex and age characteristics revealed that the prevalence of T2DM detected by screening was 15.2% among males, while it was 11.6% among females. In addition, T2DM was diagnosed in 8.0% of the patients aged under 50 years and in 12.8% of the patients aged 50 - 59 years.
When the age was considered as the only criterion for screening, T2DM was detected in only 4.2% of the patients aged under 50 years, and the prevalence of T2DM in the same age group was 12.8% when three risk factors were evaluated.
As for the patients included in the scenario with testing for glucose twice, 14% paid the first visit in a fasting state. Incidental glycemia was determined in 86% of the patients, and 23% of them had incidental glycemia levels ranging from 7.8 mmol/L to 11.1 mmol/L, which meant that their fasting glucose levels had to be re-measured later. A total of 148 patients (68.8% of this number) did not show up for a repeat examination but were referred to an endocrinologist (Appendix 3).
When the diagnosis was clarified by HbA1c assessment, the first fasting glucose determination was performed for 29% of the patients. Of these, 30% of the patients had glucose levels above 6.0 mmol/L but below 7.0 mmol/L, and only 2% of the patients had HbA1c levels above 6.5%. Out of 60 patients with fasting glycemia ≥ 7.1 mmol/L, 31 patients had additional HbA1c determinations, and 29 (94%) patients had HbA1c levels ≥ 6.5%. Thirty-two patients with incident glycemia ≥ 11.1 mmol/L had additional HbA1c determinations, and 6 (19%) patients had HbA1c levels below the diagnostic value. Out of 88 patients with incident glycemia of 7.8 - 11.1 mmol/L, only 67 (77%) patients had their HbA1c level measured (Appendix 4).
5. Discussion
T2DM increases the susceptibility to infectious diseases and worsens patient outcomes, and is also associated with significant cardiovascular morbidity and mortality (19-21). Screening asymptomatic cases of T2DM can provide early detection and treatment with improved overall patient health as well as reduce the burden of T2DM on the economy (22).
In our study, the diagnosis of T2DM was established by an endocrinologist in 9.3% of the cases with one eligibility criterion and in 15.9% of the cases with three eligibility criteria which exceed the official data regarding the prevalence of T2DM in Uzbekistan (12). Moreover, the detectability of T2DM among female patients was increased in proportion to their age. Generally, females have higher rates of T2DM in youth, whilst men have a higher prevalence in midlife (23). In our study, the prevalence of T2DM was higher among males (15.2%) than females (11.6%); however, the number of females exceeded that of males, which may have exerted an impact on the study results.
Key tools to assist in the management of patients with chronic diseases are international and national guidelines (24). It is necessary to take into account the national particularities and possibility of applying certain methods of screening, diagnostics, and treatment when the resources are limited (25-27).
Suggested strategies for screening T2DM include fasting plasma glucose and HbA1c, diabetes risk assessment using the DRS scale and various questionnaires, as well as a combination of the given strategies. A study conducted in Shanghai, China, demonstrated the feasibility of screening for T2DM by assessing fasting plasma glucose levels in the adult population (28). The authors’ specific risk assessment system included the data on age, sex, BMI, WC, systolic BP, and family history of T2DM.
Previously proposed screenings had certain disadvantages when performed in the Republic of Uzbekistan. Thus, the applicability of the FINDRISK questionnaire in the Republic of Uzbekistan was questionable (18). In addition, it is difficult for primary care physicians and nurses to fill out the questionnaires locally due to the lack of personal computers and the skills required for medical staff working in remote regions of the country.
According to the results of the previous studies conducted in the Russian Federation, the factors associated with the highest risk of developing T2DM were male sex, higher BMI, increased WC, BP, and triglyceride levels (29). Among patients with cardiovascular diseases, the prevalence of T2DM in Russia was 8.00 - 13.99%, while the same parameter among general population was 5.44%. In our region, therefore, it was reasonable to use BMI, WC, and BP as risk factors for T2DM. Taking the limited resources and the lack of medical professionals into account, only those parameters were selected in our study that were capable of easily assessing the conditions through a quick examination, including age, BP, BMI (which was calculated later), and WC.
The present study found that the prevalence of T2DM was 12.3% when patients were screened by GPs, while it was 12.6% when patients were screened by endocrinologists. Thus, the difference in the diagnosis of T2DM was 2.4% (abs. 0.3%), which indicated that T2DM screening may have been performed by GPs without the assistance of endocrinologists when appropriate educational activities were provided. Furthermore, the detectability of T2DM was discovered to increase in proportion to the age of the examined patients and the number of considered risk factors (i.e., arterial hypertension and obesity), which was consistent with data from previously conducted population studies (22, 30).
T2DM is a costly disease for low- and middle-income countries (31). Medical practice in high-income countries has shown that nearly two-thirds of new cases of T2DM can be detected before noticeable symptoms appear, but the average cost per patient, according to a 2012 study, is 377 pounds sterling (32). Such costs are reasonable and justified for the UK but not optimal in the context of low- and middle-income countries; therefore, the most rational and cost-effective screening strategy must be implemented in the given countries.
A systematic review (2019) reviewing 52 publications with information on low- and middle-income countries (mostly Asian and Latin American countries) (31) reported that the median outpatient cost per visit was seven US dollars. The median annual cost for inpatient care was 290 US dollars, and the median cost for laboratory tests was 25 US dollars. The median annual medication cost was 177 US dollars, with a particularly large variation found for insulin provision. The treatment of complications tended to be expensive but varied by country and type of complication.
In the Russian Federation, the total direct costs of the treatment for T2DM, its complications, and comorbidities per patient per year were more than 2,700 US dollars, the direct nonmedical costs were more than 600 US dollars, and the indirect costs were almost 3,900 US dollars (33). Thus, the total cost of T2DM treatment per patient per year was almost 7,300 US dollars.
In the present study, the cost of identifying one patient with T2DM by determining glucose levels twice was approximately 2.9 times (286%) cheaper than that by determining HbA1c as an additional criterion of incidental glycemia. The cost of identifying one patient with only age or three risk factors differed by 25.7%, which may have been regarded as an insignificant burden on the budget. Only 14% of the patients were examined in a fasting state, and 68.8% of the patients failed to show up for a follow-up examination. In real clinical practice, this situation is one of the reasons why patients with prediabetes and T2DM are lost when their diagnosis should be included in the medical record.
The present study faced some limitations. First, it was conducted in a clinical setting, and, therefore, the prevalence of T2DM may have been overestimated. Second, there was a lack of data about T2DM screening in the low-income countries of the region. A well-established database existed in Russia; however, the ethnic composition differed from that of the Republic of Uzbekistan. At the time of this study, third, Russia was a middle-income country while Uzbekistan was a low-middle income one. Final limitation was the methodology of cluster randomization with only four villages and cities, since the prevalence may have been different in other parts of the country. However, it should be noted that the current study reflected the results of a pilot study.
5.1. Conclusions
Due to the high prevalence of undiagnosed T2DM in the Republic of Uzbekistan, it was highly recommended that a permanent system of T2DM screening should be deployed. To create a national screening system, it was also strongly suggested that administrative tasks should be fulfilled, key performance parameters be defined, funding be allocated, and primary care physicians and nursing staff be educated about the criteria and rules for screening.