1. Background
The COVID-19 pandemic, as a serious public health problem, has put a huge burden on global health and the economy (1). This disease is associated with various disorders and complications (2). Studies have shown that in hospitalized COVID-19 patients, fluid and electrolyte disturbances occur frequently and may link to disease severity and poor prognosis (3). Some studies have found that various electrolyte disturbances are associated with the increased rate of mortality in COVID-19 patients (3). Compared to people with normal electrolyte levels, patients with lower serum concentrations of chloride, potassium, calcium, and sodium are more likely to develop the severe disease (4). Serum phosphate disorders are also common in people with severe diseases (5). Phosphate is an essential electrolyte involved in physiological processes such as energy storage, metabolism, bone mineralization, and plasma acid-base buffers. Both hypophosphatemia and hyperphosphatemia occur in different patients (6).
Hypophosphatemia can be caused by decreased intake or absorption, excessive energy consumption, increased renal excretion, or intracellular displacement leading to systemic abnormalities, including neurological disorders (seizures and mental status changes), respiratory failure due to muscle weakness, decreased myocardial contraction, arrhythmias, blood disorders, gastrointestinal disorders, and rhabdomyolysis (7, 8). In different studies, the prevalence of hypophosphatemia in critically ill patients was reported at a rate of 10% to 80% (6). Hyperphosphatemia occurs because of renal dysfunction, tumor lysis syndrome, hemolysis, rhabdomyolysis, or lactic ketoacidosis. Hyperphosphatemia is also common in the intensive care unit (ICU). Although sepsis is a contributing factor to developing acute kidney injury (AKI), it is also associated with a higher risk of hyperphosphatemia (6, 9). Abnormal serum phosphate levels are known to be predictors of adverse clinical outcomes such as increased duration of mechanical ventilation, cardiac and arrhythmic dysfunction, insulin resistance, and increased mortality (10-12). Evidence of the clinical significance of serum phosphate disorders in patients with COVID-19 is controversial (6).
2. Objectives
In this study, we investigate the association of hypophosphatemia and hypophosphatemia at admission with the mortality of COVID-19 patients.
3. Methods
This retrospective cohort study was performed between March 5, 2020, and March 21, 2021, based on the data of all COVID-19 patients registered in an academic hospital in Ilam, southwest Iran.
3.1. Inclusion and Exclusion Criteria
All consecutive hospitalized patients during the study period were included, and all the patients with incomplete and missing data (greater than 20%) in medical records were excluded from the study.
3.2. Data Collection
Baseline characteristics, medical history, and laboratory findings, including age, sex, body mass index (BMI), cigarette smoking status, intensive therapy unit, chronic comorbidities (cardiovascular disease (CVD), hypertension (HTN), chronic lung diseases, diabetes, autoimmune disease, neurologic disease, chronic kidney disease (CKD), malignancy, liver diseases), as well as various hematological test findings (phosphate level, total bilirubin, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), platelet count, white blood cells (WBCs), blood urea nitrogen (BUN), serum creatinine, hemoglobin (Hb), lactate dehydrogenize (LDH), calcium, and magnesium), were collected.
In this study, the time from admission to hospital discharge or death was considered a survival time. Serum phosphate of 2.6 - 4.5 mg/dL was considered the normal range. Hypophosphatemia was considered at a serum level less than 2.6 mg/dL, and values more than 4.5 mg/dL were considered hyperphosphatemia. The Cox proportional hazard (PH) regression analysis was performed for the effect of phosphate levels and other variables on COVID-19 mortality. For each regression variable, the scaled Schoenfeld residual was used to test the PH assumption of the Cox model. The results indicated that the main variable of the study (phosphate levels) and all other variables except Hb level satisfied the PH assumption. Variables with a P value of less than 0.2 were also considered statistically significant in univariate analyses and exported to the multivariate model. The Akaike information criterion (AIC) was used to determine the best fitting final model for the data. Cox PH regression models were used to identify the effect of phosphate levels on patients’ mortality with COVID-19 using STATA version 10 (College Station, TX: StataCorp LP). White blood cells, Hb, and platelet levels were measured directly in the Sysmex KX-21N hematology analyzer, and the blood chemistry assays (such as phosphate, magnesium, calcium, creatinine, BUN, CRP, and total bilirubin) were determined using commercial kits in the BT 3000 instrument, which were calibrated every week for measurement error.
4. Results
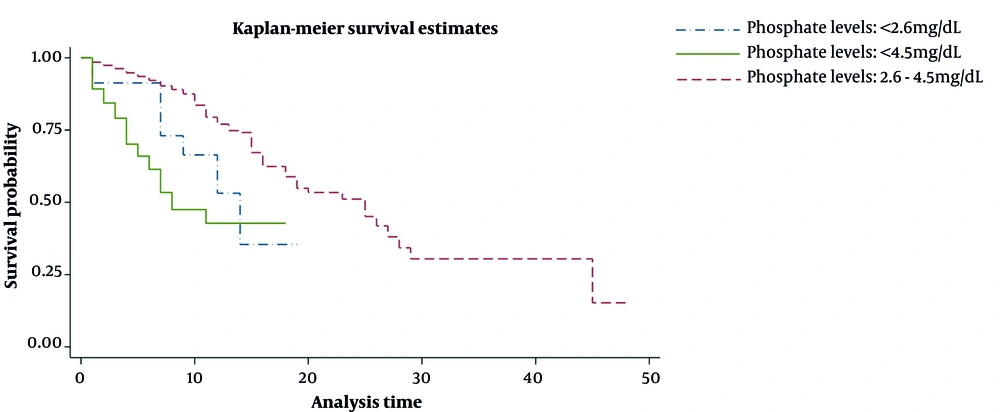
The mean age of patients who died was 65.9 (SD 1.1) years, and that of recovered patients was 55.1 (SD 0.5) years. Hypophosphatemia and hyperphosphatemia were observed in 46 (3.4%) and 65 (4.8%) patients, respectively, and the other 1238 patients (91.8%) had a normal serum phosphate level. In total, 1349 COVID-19 patients were registered, of whom 463 (34%) were admitted to the ICU, and 192 (14%) died in the hospital. In-hospital mortality in patients with hypophosphatemia and hyperphosphatemia were 24.4% and 42.4%, respectively, which was higher than the normal group rate of 12.3%. In this study, 58% of hypophosphatemia patients and 79% of hyperphosphatemia patients had at least one underlying health condition (Table 1). During hospitalization, 29% of the hyperphosphatemia group and 11% of the hypophosphatemia group had acute or chronic kidney disease. Mean LDH levels in patients with low, normal, and high serum phosphate levels were 685.4, 622.2, and 755.5 U/L, respectively. Based on the Kaplan-Meier curve, the median survival time was 14 days for hypophosphatemia and 8 days for hyperphosphatemia patients, lower than the normal group with a median survival time of 25 days (Figure 1). Cox univariate analyses revealed several risk factors, including low and high serum phosphate levels, significantly associated with COVID-19 mortality (Table 1). The unadjusted hazard ratio (HR) for hypophosphatemia was (HR, 2.24; 95% CI, 1.21 - 4.14; P = 0.01). Additionally, HR for the high phosphate level vs the normal level was 3.99 (95% CI, 2.66 - 5.99; P < 0.001; Table 1). Variables with a P value less than or equal to 0.05 in univariate analysis were then entered into a multivariate Cox PH model. The final model includes age, sex, BMI, smoking, CVD, HTN, chronic lung diseases, autoimmune disease, malignancy, intensive therapy unit, ESR, WBC, BUN, calcium, magnesium, and phosphate levels, which was the best fitted model because it had the smallest AIC (i.e., 1398.9). We had 4 interaction terms (CVD * intensive therapy unit, intensive therapy unit * autoimmune disease, BMI * hypertension, and malignancy * intensive therapy unit) to include the adjusted model. None of the interaction terms was statistically significant and removed from the adjusted model. In Table 1, explained variations show that the predicted power of the adjusted model is 60%.
| Variables c | Deaths | Survivals | Crude Hazard Ratio (95% CI) d | P Value e | Adjusted Hazard Ratio (95% CI) f | P Value g |
|---|---|---|---|---|---|---|
| Age (y) | 65.92 ± 1.13 | 55.10 ± 0.50 | 1.03 (1.02 - 1.04) | < 0.001 h | 1.01 (1.00 - 1.02) | 0.18 |
| Sex | ||||||
| Female | 65 (33.85) | 512 (44.25) | 1 i | - | 1 | - |
| Male | 127 (66.15) | 645 (55.75) | 1.64 (1.21 - 2.21) | 0.001 h | 1.43 (0.96 - 2.12) | 0.08 |
| Body mass index (kg/m2) | 26.49 ± 0.32 | 26.98 ± 0.15 | 0.96 (0.93 - 1.00) | 0.05 h | 0.96 (0.92 - 1.01) | 0.10 |
| Cigarette smoking status | ||||||
| Non-smoker | 181 (96.28) | 1109 (96.69) | 1 | - | 1 | - |
| Ex-smoker | 3 (1.60) | 7 (0.61) | 2.55 (0.81 - 8.01) | 0.11 h | 2.13 (0.51 - 8.94) | 0.30 |
| Smoker | 4 (2.13) | 31 (2.70) | 0.70 (0.26 - 1.90) | 0.49 | 0.48 (0.11 - 2.00) | 0.31 |
| Cardiovascular disease | ||||||
| No | 123 (64.40) | 924 (80.14) | 1 | - | 1 | - |
| Yes | 68 (35.60) | 229 (19.86) | 1.60 (1.19 - 2.15) | 0.002 h | 1.41(0.92 - 2.17) | 0.12 |
| Hypertension | ||||||
| No | 107 (56.02) | 814 (70.48) | 1 | - | 1 | - |
| Yes | 84 (43.98) | 341 (29.0) | 1.38 (1.03 - 1.84) | 0.03 h | 1.12 (0.73 - 1.70) | 0.61 |
| Chronic lung diseases j | ||||||
| No | 168 (87.50) | 1110 (95.94) | 1 | - | 1 | - |
| Yes | 24 (12.50) | 47 (4.06) | 2.22 (1.45 - 3.41) | < 0.001 h | 1.30 (0.77 - 2.20) | 0.33 |
| Diabetes | ||||||
| No | 127 (66.15) | 903 (78.18) | 1 | - | 1 | - |
| Yes | 65 (33.85) | 252 (21.82) | 1.37 (1.01 - 1.85) | 0.04 h | - | - |
| Autoimmune disease | ||||||
| No | 184 (96.34) | 1131 (97.75) | 1 | - | 1 | - |
| Yes | 7 (3.66) | 26 (2.25) | 1.79 (0.84 - 3.80) | 0.13 h | 2.59 (1.07 - 6.23) | 0.03 h |
| Neurologic disease | ||||||
| No | 176 (91.67) | 1112 (96.11) | 1 | - | - | - |
| Yes | 16 (8.33) | 45 (3.89) | 1.29 (0.77 - 2.16) | 0.34 | - | - |
| Chronic kidney disease | ||||||
| No | 174 (90.63) | 1108 (95.76) | 1 | - | - | - |
| Yes | 18 (9.38) | 49 (4.24) | 2.09 (1.29 - 3.41) | 0.003 h | - | - |
| Malignancy | ||||||
| No | 175 (91.15) | 1138 (98.36) | 1 | - | 1 | - |
| Yes | 17 (8.85) | 19 (1.64) | 2.12 (1.89 - 5.13) | < 0.001 h | 2.64 (1.32 - 5.26) | 0.01 h |
| Liver diseases | ||||||
| No | 190 (98.96) | 1150 (99.39) | 1 | - | - | - |
| Yes | 2(1.04) | 7 (0.61) | 1.45 (0.36 - 5.84) | 0.60 | - | - |
| Intensive therapy unit | ||||||
| No | 35 (18.23) | 976 (84.43) | 1 | - | 1 | - |
| Yes | 157 (81.77) | 306 (15.57) | 7.92 (5.45 - 11.52) | < 0.001 h | 5.77 (3.67 - 9.08) | < 0.001 h |
| Total bilirubin (mg/dL) | 0.95 ± 0.10 | 0.72 ± 0.11 | 0.99 (0.95 - 1.03) | 0.57 | - | - |
| Erythrocyte sedimentation rate (mm/h) | 42.37 ± 2.17 | 35.97 ± 0.85 | 1.00 (0.99 - 1.01) | 0.30 | 0.99 (0.99 - 1.01) | 0.57 |
| C-reactive protein | ||||||
| Negative | 20 (13.79) | 199 (21.13) | 1 | - | - | - |
| 1+ | 27 (18.62) | 204 (21.66) | 1.07 (0.60 - 1.92) | 0.81 | - | - |
| 2+ | 26 (17.93) | 394 (41.83) | 1.25 (0.70 - 2.24) | 0.46 | - | - |
| 3+ | 72 (49.66) | 757 (43.3) | 1.28 (0.78 - 2.11) | 0.34 | - | - |
| Platelet (%) k | 20.61 ± 0.66 | 21.09 ± 0.24 | 0.99 (0.97 - 1.01) | 0.22 | - | - |
| White blood cells (× 103/μL) | 14.82 ± 1.59 | 8.22 ± 0.26 | 1.01 (1.01 - 1.02) | < 0.001 h | 1.01 (1.00 - 1.02) | 0.01 h |
| Blood urea nitrogen (mg/dL) | 70.95 ± 3.49 | 38.80 ± 0.91 | 1.01 (1.00 - 1.01) | < 0.001 h | 1 (1.00 - 1.01) | 0.01 h |
| Serum creatinine (mg/dL) | 1.89 ± 0.10 | 1.28 ± 0.04 | 1.12 (1.08 - 1.17) | < 0.001 h | - | - |
| Hemoglobin (g/dL) | 12.53 ± 0.18 | 13.41 ± 0.06 | 0.91 (0.85 - 0.96) | 0.001 h | - | - |
| Lactate dehydrogenize (U/L) | 259.64 ± 13.34 | 584.20 ± 19.92 | 1.01 (1.00 - 1.02) | < 0.001 h | - | - |
| Calcium (mg/dL) | 9.27 ± 0.05 | 9.54 ± 0.02 | 0.77 (0.64 - 0.93) | 0.01 h | 0.83 (0.65 - 1.06) | 0.14 |
| Magnesium (mg/dL) | 2.25 ± 0.03 | 2.17 ± 0.01 | 1.52 (1.08 - 2.14) | 0.02 h | 0.97 (0.61 - 1.53) | 0.89 |
| Phosphate levels (mg/dL) l | ||||||
| < 2.6 | 11 (5.73) | 35 (3.03) | 2.24 (1.21 - 4.14) | 0.01 h | 2.53 (1.15 - 5.58) | 0.02 h |
| 2.6 - 4.5 | 153 (79.69) | 1085 (93.78) | 1 | - | 1 | - |
| > 4.5 | 28 (14.58) | 37 (3.20) | 3.99 (2.66 - 5.99) | < 0.001 h | 1.77 (1.00 - 3.14) | 0.05 h |
| Explained variation (R2) | 0.60 | |||||
a Values are expressed as mean ± SE or No. (%).
b The Akaike information criterion = -2 Log Likelihood + 2 P, where P is the number of parameters in the model.
c The final model includes age, sex, body mass index, smoking, cardiovascular disease, hypertension, chronic lung diseases, autoimmune disease, malignancy, intensive therapy unit, erythrocyte sedimentation rate, white blood cell, blood urea nitrogen, calcium, magnesium, and phosphate levels, which was the best fitted model because it had the smallest Akaike information criterion (i.e., 1398.9).
d Crude hazard ratio: Hazard ratio for variables in univariate analysis.
e P value for crude hazard ratio.
f Adjusted hazard ratio: Hazard ratio for variables in multivariate analysis.
g P value for adjusted hazard ratio.
h Significant.
i Reference category.
j Chronic lung diseases: Asthma and chronic obstructive pulmonary disease (COPD).
k Platelet count is calculated: Platelet/10000.
l Serum phosphate levels < 2.6 and > 4.5 mg/dL were considered hypophosphatemia and hyperphosphatemia, respectively.
In a multivariate model, the association of phosphate levels with in-hospital mortality was investigated after adjusting for various confounders (such as age, male gender, BMI, intensive therapy unit, calcium, magnesium, and ESR) and comorbidities (such as CVD, HTN, chronic lung diseases, autoimmune disease, and malignancy). In the adjusted model, a multivariate Cox PH regression analysis revealed that hypophosphatemia (adjusted HR, 3.67; 95% CI, 1.72 - 7.84; P = 0.001) and hyperphosphatemia (adjusted HR, 1.73; 95% CI, 1.01 - 3.02; P = 0.05) were associated with higher odds of COVID-19 mortality (Table 1). Our results showed that the low and high serum phosphate levels increased 3.67 times and 1.73 times death hazard compared to patients with normal serum phosphate levels. Although due to other risk factors in these patients, all 3 groups with normal, low, and high phosphate levels had an increased hazard of death, the hazard was higher in patients with high and low serum phosphate levels.
5. Discussion
This study investigated the relationship between hypo-electrolyte disorder and hyperphosphatemia and the survival of COVID-19 patients referred to Mostafa Khomeini Hospital in Ilam. The results showed that 8.2% of the total sample had phosphate electrolyte imbalance at admission, and 45% of these patients were admitted to the ICU. Previous studies have shown that COVID-19 patients had lower serum phosphate levels than healthy individuals, and severe or critical COVID-19 patients had reduced phosphate levels than moderate COVID-19 patients (13, 14). These results are consistent with the present study that deceased patients had lower serum phosphate levels than patients who did not die. The chance of death was 3.67 times higher in patients with hypophosphatemia than in patients with normal phosphate levels (P = 0.001).
Inadequate dietary intake and increased protein and energy consumption can reduce the total body phosphate stores and thus put the body at risk for hypophosphatemia (15). Various studies have shown that hypophosphatemia is linked to factors such as low protein levels, albumin, creatinine, Hb, BMI, adipose tissue index, and lean tissue index, indicating poor nutrition and anorexia in COVID-19 patients (16, 17). Systemic inflammation, followed by cytokine storms in critically ill patients, can increase energy consumption and stimulate catabolic processes, causing the release of phosphate from the intracellular pool into the extracellular space because of cell injury or cell death. This could explain the occurrence of rhabdomyolysis and most of the symptoms of chronic fatigue and myalgia in COVID-19 patients (8, 15). Other factors that may cause hypophosphatemia in COVID-19 patients include large bowel dysfunction, AKI, and respiratory alkalosis (15). Respiratory alkalosis due to hyperventilation in COVID-19 patients is associated with an increase in intracellular PH, increasing glycolysis activity, leading to the shift of phosphate from the extracellular to the cell cytoplasm (15).
This study showed a significant relationship between hyperphosphatemia and the death of COVID-19 patients. The odds ratio of death in patients with hyperphosphatemia was 1.73 times more than that of patients with normal phosphate levels (P = 0.05). However, this relationship was weaker than the relationship between hypophosphatemia and death in these patients. This finding is consistent with previous studies’ results, in which hyperphosphatemia was independently associated with in-hospital mortality (6, 18). In the study by Kuo et al., the 90-day death rate in burn ward patients was associated with hyperphosphatemia (19). In addition, in the study by Miller et al., in 197 patients who were admitted to the ICU and diagnosed with septic shock, hyperphosphatemia showed a significant relationship with death (20). Hyperphosphatemia in COVID-19 patients may be associated with other underlying diseases such as kidney disease. In this study, 29% of patients with hyperphosphatemia disorder had chronic and acute kidney disease during hospitalization. The phosphate ion releases due to cytokine-induced cell damage and may be associated with hyperphosphatemia in COVID-19 patients (21). In this study, we found a significant linear relationship (not mentioned in the study) between hyperphosphatemia and the amount of lactate dehydrogenized, which can be explained in the form of cell damage and increased serum phosphate (22).
This study has limitations, including the fact that we assessed hypo- and hyperphosphatemia using serum phosphate data at the time of admission to the hospital, which may be corrected during treatment under the influence of treatment and patient survival. In addition, errors may occur during sampling and measuring serum phosphate levels. The study was also conducted at a COVID-19 patient registration center, and the results may not be generalizable to other populations.
5.1. Conclusions
Hypo- and hyperphosphatemia in COVID-19 patients were associated with increased in-hospital mortality. Early diagnosis and good treatment management are needed to reduce the harmful effects of serum phosphate imbalance and improve patient outcomes.