1. Introduction
Ganglioneuromas (GNs) are rare benign tumors from the autonomic nervous system, composed of ganglion and Schwann cells. Ganglioneuromas may develop ubiquitously in the human body, although they are most frequently located in the abdomen, in particular, adrenal glands and retroperitoneum. Less frequent localizations are the central nervous system, the heart, the bones, and the intestine. Other sites, such as the cervical region, are unusual. Ganglioneuromas may be asymptomatic or may cause pressure symptoms on the surrounding tissues. They can be solitary or associated with type 1 neurofibromatosis, multiple endocrine neoplasia type 2B, or Turner syndrome.
In this article, we report a rare case of GN of the neck presenting in a four-year-old girl, discuss the differential diagnosis and main clinical, imaging, and pathological features of GNs, and review the cases of pediatric GNs so far described in the literature.
2. Case Presentation
A four-year-old female was referred to the Endocrine Unit due to a slow-growing mass at the level of the left thyroid lobe. No history of the disease was present in her medical record, and she was not under any drug. Family history was unremarkable. Her mother had observed a cervical mass progressively enlarging in the past two years with no apparent pressure symptoms such as dyspnea or dysphagia.
On palpation, a smooth mass of hard consistency, mobile on swallowing, was felt in the anterior neck, inferiorly to the left of the trachea. Lab tests detected normal blood count, liver renal, and coagulation function values. Serum Thyrotropin (TSH) concentrations were 5.14 mcIU/mL (normal range (n.r.): 0.35 – 4.94), FT4 8.8 pg/mL (n.r.: 6 - 12), calcitonin < 1 pg/mL, calcium 10.4 mg/dL (n.r: 8.5 - 10.5), and parathormone (PTH) 26 pg/mL (n.r.: 10 – 70).
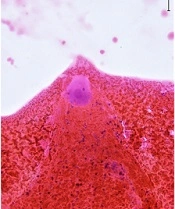
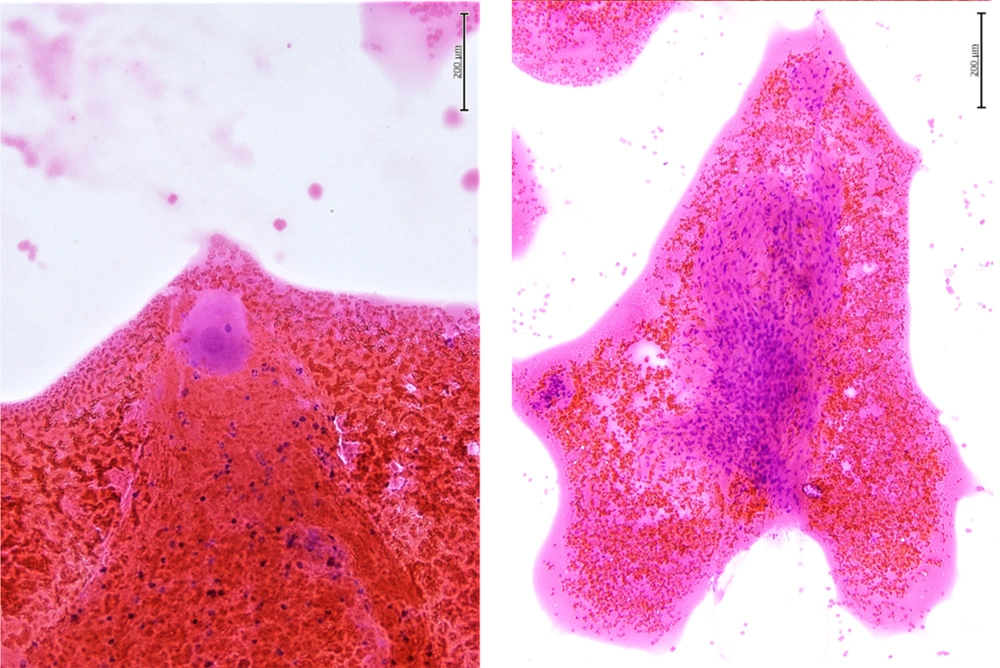
The neck ultrasound showed a solitary hypoechoic, mildly vascularized, 2.1 × 2.3 × 3.3 cm large nodule with well-defined margins close to the inferior third of the left thyroid lobe; no concurrent suspicious lymph nodes were seen. Fine needle aspiration biopsy was performed: Cytological exam (Figure 1A-B) demonstrated a population of large cells with eosinophilic cytoplasm, regular nuclei, and prominent nucleoli and spindle cells without significant atypia, consistent with a benign lesion of neurogenic origin. The wash-out liquid was negative for parathormone, calcitonin, and thyroglobulin.
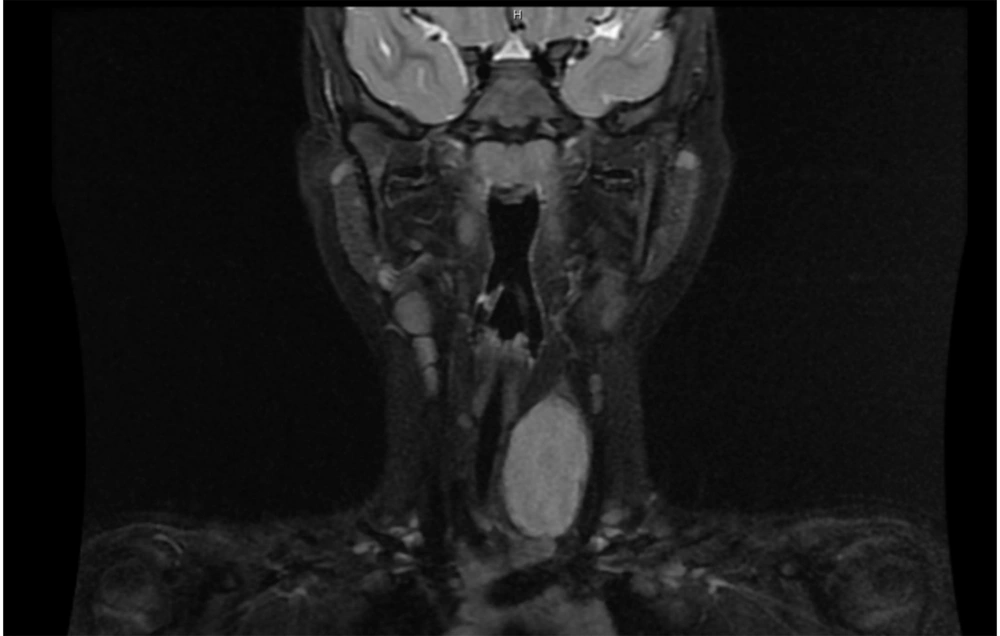
Magnetic resonance imaging (MRI) of the neck (Figure 2) revealed a well-circumscribed 4 cm large mass, closely adhering to the lower third of the left thyroid lobe. The neoplasm dislocated the trachea contralaterally and showed a strong signal in T2 sequences enhancing after contrast administration. No cervical lymphadenopathy was evident. The report suggested a lesion originating from the peripheral nervous system.
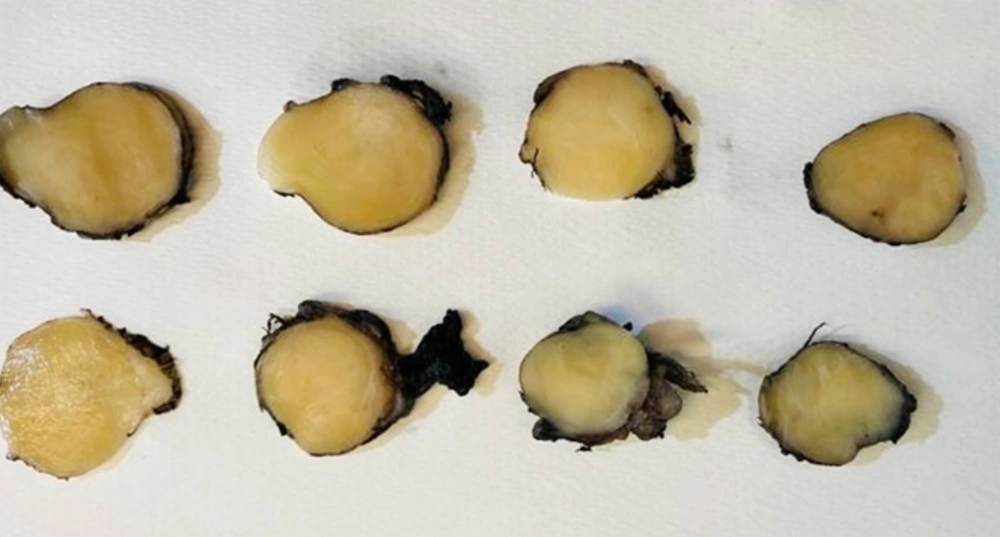
Subsequently, the patient underwent surgery. The complete excision of the neoplasm was achieved without complications: in particular, there was neither evidence of Horner syndrome symptoms nor voice changes. Grossly (Figure 3), the mass measured 2 × 2 × 3 cm and appeared semi-capsulated, with defined margins and a white, compact cut surface. Histological examination, completed after fixation with formalin (4 µm sections were obtained and stained with hematoxylin and eosin (H&E) after deparaffinization), showed a thin-capsulated lesion with well-defined borders and moderate cellularity. The cell population comprised two subtypes: Spindle cells arranged in small intersecting fascicles separated by loose stroma and consistent with Schwann cells and large elements with abundant cytoplasm, round to oval vesicular nuclei, and prominent nucleoli corresponding to ganglion cells. Schwann cells stained positively for S-100 protein, ganglion cells, and their axonal processes for synaptophysin, chromogranin A, and neurofilament (NF) protein. The NF expression and histochemical staining for AB-PAS showed the presence of cellular neuropil all over the neoplasm. Staining for cytokeratins, EMA, and TTF-1 was negative. The Mib-1 and Ki67 label indexes were less than 5%. The diagnosis of ganglioneuroma (GN) of the neck with benign biological behavior was made. The presence of neuroblasts was sought by thorough sampling and was excluded. Interestingly, the lesion bordered normal thyroid tissue and a fragment of the normal parathyroid gland, thus confirming the proximity between the neoplasm and the thyroid.
Genomic DNA was extracted from peripheral blood leukocytes using standard procedures with DNeasy Blood Kit on the MagCore Extraction System. Molecular characterization of DNA was performed by NGS, using NGS Clinical Exome Sequencing (CES), which comprises 4800 genes using Sophia Genetics Kit on the NextSeq550 platform (Illumina, San Diego, CA, USA), according to the manufacturer's protocol. The BaseSpace pipeline (Illumina, https://basespace.illumina.com/) and the SOPHIA DDM software were used for the variant calling and annotating variants, respectively. Variants identified were visualized by the Integrative Genome Viewer (IGV). The BWA alignment algorithm was used to map sequence reads to the UCSC human genome reference build 19. Variants altering the coding sequence were selected when present at a frequency of < 1:100 (0.01) in the control population. Sequence changes were analyzed and interpreted, taking into consideration cancer-related genes. The clinical significance of the variants identified was examined using standards and guidelines recommended by the American College of Medical Genetics (ACMG) and the Association for Molecular Pathology (AMP), integrated with Invitae Variant Classification Sherloc Criteria (1, 2).
We performed genetic analysis using the CES approach and focused on evaluating genes associated with predisposing conditions to tumors and genes associated with somatic cancer. No pathogenic variants were detected in paraganglioma-associated genes (FGFR1, FGFR3, LZTR1, MGMT, NFKBIA, PPARG, TACC3, TP53, IDH1, ERBB2, PTEN, EGFR, NF1, NF2, BRCA2, POT1, PIK3CA, CDK4, MDM2, ALK, ACVR1, MLH1, MSH2, EPCAM, PMS2, MSH6, MLH3, BRAF, KIF1B, VHL, APC, RET, SDHA, SDHB, SDHC, SDHD, TMEM127, MAX, SDHAF2, KIT, PDGFRA, CHEK2, ATM, DLST, and SLC25A11) but we identified 14 germline Variants of Uncertain Significance (VUS) affecting 14 different genes (Table 1).
| Gene | Germinal Result | Variant Type | VF % | Condition | gnomAD Variant Frequency |
|---|---|---|---|---|---|
| PALB2 | NM_024675: c.1010T>C (rs45494092) p.(Leu337Ser) | Missense | 36 | # 114480 | 0.01456 |
| FOXE1 | NM_004473.4: c.511_514delins p.(Ala171_Ala172delinsProPro) | Missense | 50 | # 616534 | |
| FOXG1 | NM_005249: c.248A>C p.(Gln83Pro) | Missense | 30.8 | # 613454 | |
| SAMD9 | NM_017654: c.2642A>G (rs140921998) p.(Asp881Gly) | Missense | 31 | # 610455 | 0.01617 |
| PMS2 | NM_000535: c.1437C>G (rs63750685) p.(His479Gln) | Missense | 45.4 | # 619101 | 0.005482 |
| SLX4 | NM_032444: c.2824G>C (rs114014006) p.(Glu942Gln) | Missense | 59 | # 613951 | 0.01758 |
| TET2 | NM_001127208: c.5167C>T (rs146348065) p.(Pro1723Ser) | Missense | 66.7 | # 614286 | 0.006599 |
| SMO | NM_005631: c.518G>A (rs147491841) p.(Arg173His) | Missense | 41.6 | % 605462 | 0.0006374 |
| TSC1 | NM_000368: c.3103G>A (rs118203742) p.(Gly1035Ser) | Missense | 52.5 | # 191100 | 0.00138 |
| RB1 | NM_000321: c.1988A>G (rs1007286459) p.(Asn663Ser) | Missense | 48.9 | # 180200 | 0.00001315 |
| CASP10 | NM_032977: c.1228G>A (rs13010627) p.(Val410Ile) | Missense | 43 | # 603909 | 0.04197 |
| RASA1 | NM_002890: c.296C>T (rs111840875) p.(Ala99Val) | Missense | 47.3 | % 605462 | 0.02007 |
| FANCA | NM_000135: c.3412C>G (rs138417003) p.(Leu1138Val) | Missense | 51.2 | # 227650 | 0.0006963 |
| JAK2 | NM_004972: c.143G>A (rs143227399) p.(Gly48Glu) | Missense | 54.7 | # 600880 | 0.0002631 |
3. Discussion
Here, we report the case of a 4-year-old patient presenting with a massive neoplasm in the inferior neck, indistinguishable from a nodule of thyroid origin on a clinical and ultrasound basis. Although the MRI report oriented towards a lesion of neurogenic origin due to typical hyperintense appearance in T2-weighted images, fine needle aspiration cytology and the measurement of thyroglobulin, calcitonin, and parathormone on the wash-out liquid guided the final diagnosis and were crucial to the surgical decision. Indeed, undetectable thyroglobulin and calcitonin levels in the aspiration fluid allowed to exclude the origin from thyroid follicular and parafollicular cells, and undetectable parathormone concentrations allowed to rule out the parathyroid derivation.
At that point, cytological findings were essential for differential diagnosis. Fine needle aspiration biopsy yielded a material including a biphasic spindle and large cell population without nuclear atypia, of likely neurogenic origin. Thus, cytological, clinical, and imaging features were consistent with a benign neoplasm. Histological examination confirmed this hypothesis and was diagnostic for GN. As known, GNs are benign tumors that arise from the autonomic nervous system and are usually located in the posterior mediastinum or the retroperitoneum. However, they may occasionally be found in the cervical region (3, 4).
Grossly, GNs present as well-circumscribed masses, with dimensions ranging from 8 to more than 15 cm. Their cut surface is gray-white or tan-yellow, firm, with a resilient texture, and may have a trabecular or whorled appearance. On light microscopy, GNs show both ganglion cells and Schwann cells. Ganglion cells may be "mature," with dense and eosinophilic cytoplasm with distinct cell borders. The nucleus can be single, located peripherical, or present with prominent nucleolus or "dysmorphic," with even multiple or a solitary pyknotic nucleus. At the immunohistochemical examination, GNs show positive reactions for neuron-specific markers (S-100 protein, synaptophysin, NF) (5).
Differential diagnosis includes several spindle cell lesions of either primary thyroid gland origin or extrathyroidal origin, mainly Solitary Fibrous Tumor (SFT), Peripheral Nerve Sheath Tumor (PNST), and paraganglioma (PG) (6). As a ubiquitous benign neoplasm of likely mesenchymal origin, SFT occurs very rarely in children. Microscopically, SFT is classically defined by a proliferation of spindle cells, with a fibroblast-like appearance often displaying a disorganized distribution and staining positively for bcl-2, CD99, and vimentin but negatively for neuron-specific markers. Also, PNST arises from sympathetic and parasympathetic nerves and may be benign or malignant. It can develop in any body site, including the neck.
Moreover, PNST may arise from the nerves close to the thyroid area or medium to large nerves next to the thyroid capsule mimicking a primary thyroid tumor. As in the case of our patient, most subjects present with a mobile, non-tender mass in the anterior neck and do not show pressure symptoms. On light microscopy, PNST typically exhibits Antoni A and Antony B patterns, palisading of cells and Verocay bodies, and lack of ganglion cell component. In addition, PGs are rare tumors of neuroendocrine origin derived from the paraganglia of the autonomic nervous system (7). Typically, on light microscopy, PGs demonstrate a nesting pattern ("Zellballen") composed of large cells intensely positive for synaptophysin, neuron-specific enolase, and chromogranin A, while sustentacular cells are characterized by positivity for S-100 protein.
The most difficult challenge in dealing with GNs is to differentiate them from immature ganglioneuromas and ganglioneuroblastomas, as the prognosis is invariably worse in the latter. Immature ganglioneuroma includes clusters of differentiating neuroblasts or maturing ganglion cells that do not form nests, unlike ganglioneuroblastoma (5). Table 2 summarizes the cases of cervical GNs reported in the literature.
| Sex | Age, y | Side | Presenting Signs and Symptoms | Surgical Complications | Size, cm | Year | References | |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 13 | R | Neck swelling- Dysphagia | Temporary Horner | Not reported | 2008 | (8) |
| 2 | F | 1.6 | L | Neck swelling | None | 3 | 2016 | (9) |
| 3 | M | 17 | R | Pain-Dysphagia | Pain at the surgical site | 8.6 | 2018 | (10) |
| 4 | F | 5 | R | Mass | Horner Syndrome | 4 | 2008 | (11) |
| 5 | F | 7 | R | Mass | None | Non-reported | 1935 | (12) |
| 6 | F | 10 | L | Neck swelling – Hoarseness | Asymptomatic vocal cord and hypoglossal nerve palsy | 5 | 2016 | (13) |
| 7 | F | 8 | L | Neck swelling | None | 5 | 2011 | (14) |
| 8 | F | 4 | L | Neck swelling | Unknown | 6.5 | 2021 | (15) |
| 9 | M | 4 | L | Mass | None | 5 | 2020 | (16) |
| 10 | M | 7 | L | Mass | None | 7 | 2013 | (17) |
| 11 | F | 4 | R | Snoring | Right Eye myosis | 10 | 2012 | (18) |
| 12 | M | 12 | L | Mass | Horner Syndrome | 4 | 2019 | (3) |
| 13 | F | 11 | L | Mass | None | 10 | 2014 | (19) |
| 14 | F | 7 | L | Snoring | Postoperative fever, Dysphagia, Horner Syndrome | 7 | 2017 | (20) |
| 15 | F | 10 | R | Snoring | None | 15 a | 2018 | (21) |
| 16 | F | 4 | L | Neck swelling | None | 3 | Present case | - |
Abbreviations: L, left; R, right.
a The dimension is the sum of two adjacent ganglioneuromas
Sixteen patients affected by GNs of the neck have been described, including the present one. Of them, 12 (75%) were females, and six (25%) were males, aged between 1.6 and 17 years (mean age: 7.8 years). In 10 out of 16 (62.5%) patients, the mass was located in the left side of the neck and was symptomatic, mainly causing neck swelling and snoring; pain, dysphagia, and hoarseness occurred in a minority of cases. The mean largest GN diameter size was 7.4 cm (range: 3 - 10 cm). Post-surgical complications developed in half of the cases (8/16, 50%), the most frequent being Horner syndrome. Thus, the interesting issues of the case we have reported here are the following.
(1) The age of our patient was younger than the usual age of GN cases.
(2) The child did not complain of any symptoms. It was the mother who noticed the lump in the neck and requested specialist advice.
(3) The mass mimicked a thyroid nodule, and a multidisciplinary approach including ultrasound and MRI imaging, fine needle aspiration cytology, and hormone measurement on wash-out liquid could rule out the thyroid origin. A correct diagnosis is crucial to guide surgery, the mainstay therapy for GNs, and reduce the risk of complications. The accurate surgical excision of the mass allowed our patient to preserve thyroid function, avoiding post-surgical complications.
(4) Genetic testing did not evidence any pathogenetic variation associated with paraganglioma.
In conclusion, we have reported a rare case of GN of the neck, discussing clinical, imaging, pathological features, and the primary differential diagnosis and finally reviewing the cervical NG cases reported in the literature.