1. Context
Most clinicians who treat patients with hypothyroidism have encountered patients who despite achieving normal thyroid biochemistry on levothyroxine (L-T4) replacement therapy, complain of persistent symptoms and wish to be treated with liothyronine (L-T3)-containing treatments (1). The evidence from randomised controlled trials has shown no superiority of combination L-T3 + L-T4 therapy compared to L-T4 alone (2), although shortcomings in study design and the short half-life of L-T3 were potential confounders. Anecdotally some patients describe the effects of L-T3-containing treatments as “miraculous” (3). This phenomenon may be due to a placebo effect, patient perceptions that relate to the medical consultation experience, the differential effects of exogenous L-T4 on type 2 iodothyronine deiodinase (DIO2) ubiquitination between peripheral and hypothalamic-pituitary tissues (4), or genetic susceptibility to impaired efficacy of L-T4 in some hypothyroid patients,1 leading to hypothyroidism at tissue level (“low tissue T3 hypothesis”) (1). Patient requests for L-T3-containing treatments is perceived by physicians as a common barrier to appropriate thyroid hormone management (5). The position of professional organisations like the European Thyroid Association in relation to the use of L-T3 + L-T4 combination treatment has been ambivalent and seems to have done little to resolve the controversy (3).
Understanding why some hypothyroid patients remain unsatisfied with L-T4 treatment requires consideration of all the possible contributors, including physiological mechanisms of thyroid hormone actions, comorbidities, the effects of autoimmune inflammation, medically unexplained symptoms, psychosocial influences and the impact of the medical consultation (1). What has been neglected in this debate, is that thyrotoxicosis may enhance physical and mental well-being for some patients and may be a plausible explanation for why some patients prefer overtreatment with thyroid hormones (Table 1). Recognising, documenting and understanding patient experiences and perceptions of well-being associated with thyrotoxicosis is therefore important. It can provide useful insights into the mechanisms by which somatic, emotional and cognitive processes are affected in thyrotoxicosis, may explain why a minority of patients with hypothyroidism pursue overtreatment with thyroid hormones (1), and why some patients with thyrotoxicosis do not adhere to taking their anti-thyroid medication.
| Variables | Improved Features | Source/Type of Study | ||
|---|---|---|---|---|
| Somatic | Cognitive | Mood | ||
| Hyperthyroidism | + | + | Observational case control study (6) | |
| + | Observational case control study (7) | |||
| Euthyroidism treated with excess thyroid hormones | + | Observational studies and small randomised study (reviewed in (8)) | ||
| + | Low quality randomised study and anecdotal reports (9-13) | |||
| + | Prospective observational study of healthy subjects treated with L-T4 (14) | |||
| Hypothyroidism treated with excess thyroid hormones | + | + | Randomised double-blind cross-over study using L-T4 (15) | |
| + | + | + | Online patient survey (16) | |
| + | Single-blind cohort study in elderly patients (17) | |||
The terms “thyrotoxicosis” and “hyperthyroidism”, are often used interchangeably, however strictly speaking “thyrotoxicosis “refers to elevated serum thyroid hormone concentrations whatever the cause, while “hyperthyroidism” implies increased synthesis of thyroid hormones, as in Graves’ disease or toxic nodular goitre. This distinction will be adhered to in this review.
Patients with hyperthyroidism usually feel unwell (18). The symptoms vary in severity between individuals with only a modest correlation between symptoms and extent of biochemical disturbance (19), age (20) and comorbidities (21). Young patients generally tolerate hyperthyroidism better than the elderly (20). Most symptoms improve after treatment, though some may persist beyond restoration of the euthyroid state (22).
The patient experience of thyrotoxicosis has been described and captured by a variety of methods that generally include documentation and appraisal of somatic, emotional and cognitive symptoms (22, 23). Other parameters such as levels of energy or general well-being have been mentioned, but not defined or documented in detail. Quality of life (QOL) is a concept that embraces some or all of the above, and disease-specific validated tools are available, however they usually report on overall scores derived from multiple domains (2). Thus some positive experiences may be overshadowed by negative ones. In societies where overweight and obesity are prevalent, weight loss (a common sequel of thyrotoxicosis) is often a welcome symptom and can influence positively the overall experience as perceived by patients (24). In order to explore this further, we have focused on evidence that pertains to somatic symptoms, mood, cognition and body weight.
2. Evidence Acquisition
A comprehensive literature search in PubMed and Google Scholar, was performed to identify studies investigating factors influencing well-being, mood, and psychological features associated with thyroid disease. The key words used were “thyrotoxicosis”, “hyperthyroidism”, “hypothyroidism” in combination with “well-being”, “quality of life”, “mood”, “euphoria”, “psychological”, “psychometric”, “cognitive”, “physical”, “somatic”. Over 2000 results were retrieved, which were further filtered for human, adult subjects, English language and for having an abstract, which led to 577 publications. Of these, 11 studies contained primary data that were relevant to the scope of this review (Table 1).
3. Results
3.1. Well-Being and Hyperthyroidism
Several studies have reported on somatic symptoms, cognition, mood and general well-being of cohorts of patients with hyperthyroidism, studied at baseline and at various times after treatment (22, 23, 25-27). Such patients generally complain of negative symptoms associated with thyrotoxicosis, which improve with treatment, although some may persist when euthyroidism has been reached (28). All these studies (22, 23, 25-27) have reported overall mean group outcomes of their cohorts rather than individual responses, so it is possible that opposite experiences by a minority of patients may have been overshadowed by the overall trend of the group.
The authors’ anecdotal experience is that some patients may feel better when hyperthyroid than euthyroid (2). This tends to be seen in younger age groups with biochemically mild to moderate hyperthyroidism. Such patients describe having boundless energy, are particularly appreciative of reaching a desirable lower body weight than before the onset of hyperthyroidism without lifestyle changes, and display and report an improved mood.
Some evidence from population studies supports the concept of enhanced well-being in patients with hyperthyroidism. One study found that previously undiagnosed individuals with hyperthyroidism (cause of hyperthyroidism not disclosed), had fewer complaints than euthyroid controls (6). In particular, among 38 symptoms in the von Zerssen’s complaints scale (29, 30) some were significantly less frequent among hyperthyroid subjects compared to euthyroid controls (heavy or restless legs, sleeplessness, anxiety, abdominal pain, and rumination) (6). In the same study, hyperthyroid patients showed a trend towards less depression and more energy than euthyroid controls (6). Another study of previously undiagnosed patients with subclinical hyperthyroidism (defined as low serum TSH associated with normal free T3 and free T4 levels due to a variety of causes, mostly non-autoimmune) found that their mood was rated better and they were less “touchy” than controls (7). Thus, the experience from patients with hyperthyroidism is limited and poorly described, but positive effects on mood, and fewer negative somatic (e.g. heavy legs, abdominal pain) and psychological symptoms (e.g. anxiety and rumination) than in euthyroid controls have been described (6, 7).
3.2. Well-Being in Euthyroid Subjects Overtreated with Thyroid Hormones
Thyroid hormones are used inappropriately in patients without thyroid disease, because of perceived or real benefits. In an era when reliable biochemical tests of thyroid status had not yet been developed, the term “thyroid addiction” was used to describe patients consuming large amounts of thyroid hormones (31, 32), among them a woman who used thyroid extract for “slimming” and as a “tonic”, and a man who “indulged in the pleasures of the table to a greater extent without putting on weight grossly, if he takes thyroid extract” (31). Thyroid hormone containing “pep pills” were popular in the past (33) and “thyroid hormone abuse” (consumption of thyroid hormones for weight loss, or to improve energy) continues today occasionally with catastrophic consequences (34). Indeed recent evidence from several European countries suggests that thyroid hormones are still used by a minority of physicians (2.2 - 8%) in euthyroid patients for weight loss (35-50).
A long-established tradition in psychiatry is the use of L-T3 in large doses in euthyroid patients (often inducing thyrotoxicosis), as an adjunctive treatment for depression resistant to conventional treatments (8). This suggests a positive effect of L-T3 on mood in some patients with depression. Large doses of L-T3 (94 to 150 mcg daily) are advocated and promoted by some practitioners as a treatment for euthyroid patients with fibromyalgia, apparently leading to improvement of symptoms (9). Intermittent use of L-T3 by euthyroid elite athletes appears to be widespread (10), suggesting an enhancing effect on aspects of physical performance. Euthyroid body builders use thyroid hormones (51) for rapid weight loss, particularly aiming to reduce subcutaneous fat and exaggerate the appearance of the musculature.
A small double-blind placebo controlled study of L-T4 treatment (100 mcg daily) in euthyroid patients with symptoms suggestive of hypothyroidism, showed no significant benefits in physical and psychological well-being and cognitive function (52), but the dose of L-T4 was relatively low and insufficient to induce thyrotoxicosis. A prospective observational study of 29 healthy subjects treated with a large dose of L-T4 (250 mcg daily) for 8 weeks resulting in a rise of mean FT3 levels from 4.7 pmol/L to 7.4 pmol/L (normal range 3.2 - 7.2 pmol/L), showed significant improvement in memory (14).
The evidence from use of thyroid hormones in euthyroid subjects is scanty and generally of limited quality, but suggests that it is principally associated with the use of L-T3 rather than L-T4, is driven by perceived benefits on mood, weight control, general well-being and improved physical performance.
3.3. Enhanced Well-Being in Hypothyroid Subjects Overtreated with Thyroid Hormones
3.3.1. Overtreatment with L-T4
A randomised double-blind cross-over trial using L-T4 in supraphysiological doses that induced biochemical subclinical thyrotoxicosis, showed no significant difference in QOL, mood or cognition, compared to the biochemically euthyroid state (53). Another randomised double-blind cross-over study showed that subclinical thyrotoxicosis using L-T4 resulted in a mixed picture (54); while somatic symptoms were slightly worse during subclinical thyrotoxicosis compared to euthyroidism, some measures of mental health (confusion, depression, tension, motor learning and mood) improved. A prospective, single-blinded, placebo-controlled, parallel-group trial showed no impact on QOL in patients on L-T4-induced subclinical thyrotoxicosis who then transitioned to biochemical euthyroidism after L-T4 dose adjustment (55).
A single-blinded observational study in a small cohort (n = 24) of patients over 65 years old whose L-T4 dose was increased by 12.5 mcg for 3 months (resulting in a decline in serum TSH from 1.95 to 0.47 mU/L, normal reference range 0.3 - 4.0 mU/L), was associated with an improvement in mood assessed by the Geriatric Depression Scale (17).
3.3.2. Overtreatment with L-T3 and DTE
There are no randomised controlled trials comparing patients overtreated with L-T3 or DTE, to patients achieving biochemical euthyroidism with L-T3, DTE or L-T4. The evidence that L-T3 or DTE overtreatment in hypothyroidism is associated with enhanced well-being is anecdotal (56). Online patient surveys have been published in recent years and provide some insight on overtreatment with thyroid hormones, especially L-T3 and DTE. These studies recruited selected patients (users of social media, members of thyroid patient groups and blogs, who tend to be dissatisfied with L-T4 treatment and are mainly from north-western Europe and North America (3, 16, 57-59). Combination treatment with L-T4 + L-T3 was used by 9 - 43% and DTE by 13 - 50% of participants (3, 16, 57-59). Overtreatment was common with 28% of respondents admitting to adjusting the dose of thyroid hormones themselves and 14% self-reporting a suppressed serum TSH of < 0.01 mU/l (3). Symptoms attributed to hypothyroidism that seemed to cause most concern to patients in these surveys were weight gain, fatigue, low mood, and impaired memory (16), and these tended to be the symptoms reported to respond to L-T3 and DTE. A UK-based study showed that QOL was better in those taking DTE or using L-T4 + L-T3 combination therapy, than in patients treated with L-T4 (57). However, in multivariate analyses significant correlations with satisfaction and QOL were only found for age, gender, and various characteristics of the patients’ diagnosis and interaction with their physician (57). A Danish online survey of hypothyroid patients reported that combination treatment with L-T4 + L-T3 or DTE resulted in “miraculous” improvement in symptoms and well-being in 19% of patients, while overall positive effects were described by 84% of respondents (3).
3.4. Putative Mechanisms Mediating Enhanced Well-Being in Thyrotoxicosis
3.4.1. Somatic Effects
There are no data on the mechanisms by which the thyrotoxic state may mediate positive somatic experiences, a phenomenon that is in any case poorly documented (6), but may be the same as for physical performance and body weight (discussed below).
3.4.2. Physical Performance
If overtreatment with thyroid hormones enhances physical performance, then it may contribute to a sense of well-being. A disturbance of the tightly genetically determined set point (60) of the hypothalamic-pituitary-thyroid axis has been described associated with intensive training (61). Ingestion of thyroid hormones allegedly helps recovery, and hence subsequent physical performance, but evidence for this is lacking (62).
3.4.3. Effects on Body Weight
Thyroid hormones increase metabolic cycles and ion leaks across cell membranes. These direct effects of T3 via its receptors lead to increased ATP utilization (63) and negative energy balance. Several additional effects of thyroid hormones are thought to be involved in regulation of energy expenditure including activation of the PI3K pathway, mitochondrial biogenesis and mitochondrial uncoupling (64), which may lead to weight loss. A Dutch observational study of treated hypothyroid patients has shown a negative correlation between body mass index and QOL (65). In populations where obesity is common and generally perceived as undesirable, successful weight control interventions in patients with obesity are associated with improved QOL (24), suggesting a potential link between the metabolic effects of thyrotoxicosis, weight loss, improved QOL and hence sense of well-being.
3.4.4. Effects on Mood
The beneficial effect of LT3 in resistant depression has been attributed to increased sensitivity and transcription of serotonin in the brain (66). Another hypothesis proposes that intracellular T3 is low in depression, and that this relationship is causal (67), although direct evidence for either of these hypotheses is lacking.
3.4.5. Effects on Cognition
The mechanisms for enhanced cognitive function in thyrotoxicosis are unclear, but some neuroimaging changes that may support such effects have been described. Functional MRI has shown increased connectivity in temporal lobe structures of healthy volunteers rendered thyrotoxic with excessive thyroid hormone ingestion (68). While such effects may be viewed as desirable in the short-term, a body of evidence indicates associations between thyrotoxicosis and cognitive impairment (69).
4. Discussion
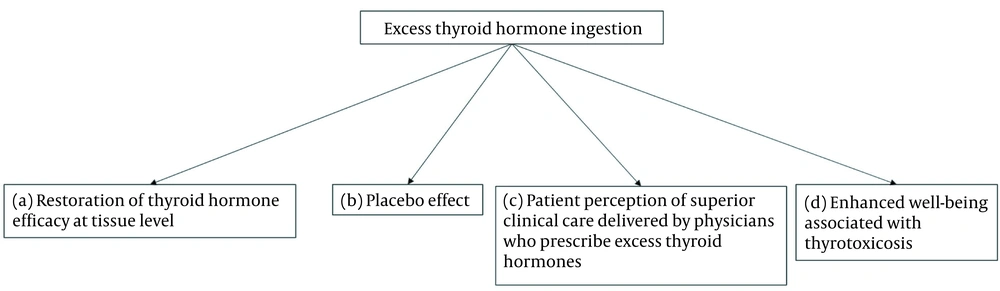
Lack of efficacy of L-T4 for some patient subgroups1 has been extensively explored as a reason for patient dissatisfaction. The transformative effects of L-T3-containing treatments reported anecdotally, but not confirmed by randomised controlled trials, may be explained by restoration of thyroid hormone efficacy at tissue level, placebo effects, or by patient perceptions of superior clinical care delivered by physicians who prescribe excess thyroid hormones (1). An additional but neglected explanation may be enhanced well-being induced by thyrotoxicosis (Figure 1). The available data suggest that this entity exists, although the evidence is weak. The principal domain that is influenced seems to be mood (Table 1). Some somatic symptoms and cognitive parameters may also be perceived by patients as being improved in the thyrotoxic state compared to euthyroidism. These effects are observed in subjects overtreated with thyroid hormones, especially with L-T3, as well as in hyperthyroidism.
Possible mechanisms by which overtreatment with thyroid hormones improve patient symptoms. (a) Improved thyroid hormone efficacy by overcoming “defects” in thyroid hormone transport or metabolism (70, 71); (b) placebo effect (2); (c) patient perception of superior clinical care delivered by physicians who prescribe excess thyroid hormones (16, 57); (d) enhanced well-being associated with thyrotoxicosis.
At present enhanced well-being associated with thyrotoxicosis should be viewed as a hypothesis. For a hypothesis to be valid, it must be scientifically testable. One of the challenges is discriminating between pharmacological effects of thyroid hormones and normal physiology at tissue level. This will require use of validated psychometric tests that can distinguish enhanced well-being from normality. In addition, serial psychometric, clinical and biochemical assessments made before and after the onset of thyroid dysfunction and at several points after the introduction of treatments. Assuming that the above is feasible, and that robust evidence confirms enhanced well-being associated with thyrotoxicosis as a real entity, it may be possible to answer several other relevant questions (Box 1).
| Questions |
|---|
| Prevalence of enhanced well-being among thyrotoxic patients. |
| Predisposition of patient characteristics (demographics, cause of thyrotoxicosis, comorbidities) to the perception of enhanced well-being. |
| Relationship of severity and duration of thyrotoxicosis with enhanced well-being. |
| Interaction of opposing patient experiences (for example, improved mood, but worse somatic symptoms) and how they influence overall patient perception of enhanced well-being. |
| Contribution of amelioration of certain pre-morbid symptoms that are unrelated to thyrotoxicosis (for example fatigue) rather than a sense of heightened well-being, to the perception of enhanced well-being. |
| Contribution of some of the negative symptoms experienced by patients due to the medication used to correct the thyroid dysfunction, to the overall patient perception of enhanced well-being. |
An important consideration is whether patients who may experience enhanced well-being while thyrotoxic who wish to maintain that state, are susceptible to harm. It can be argued that if patients are happier in the thyrotoxic state, why intervene? Thyrotoxicosis, independently of aetiology, is unequivocally associated with increased morbidity and mortality (72) and therefore treatment should be offered. Treating subclinical thyrotoxicosis is more controversial. Although associations between subclinical hyperthyroidism and poor long-term outcomes (cardiovascular morbidity and mortality, osteoporotic fractures, dementia) are well established (69, 73-76), overall benefit from interventions that restore euthyroidism (all of which have potentially harmful side-effects) has not yet been demonstrated (77). Subclinical thyrotoxicosis as a result of overtreatment with thyroid hormones is also associated with increased morbidity and mortality (72), especially when the serum TSH is suppressed to < 0.1 mu/L (78), which should be addressed by adjustment of the dose of thyroid hormones. However straightforward this line of thinking may seem, in practice enhanced well-being associated with thyrotoxicosis poses some important ethical dilemmas. How and who should judge where the balance lies between harm and benefit, when harm from denial of treatment may equate to disability, while benefit from treatment may be prevention of premature death? And how does the above apply to individuals who do not even have thyroid disease, but seek thyroid hormone treatment?
The concept of enhanced well-being associated with thyrotoxicosis is of relevance in daily clinical practice. Patients in general, and thyroid patients in particular (57, 79), long for information about their disease and their treatment. Good quality information empowers patients and improves clinical decision-making. Awareness of enhanced well-being associated with thyrotoxicosis can help both physician and patient understand the effects of treatment or disease on symptoms and can facilitate appropriate management decisions. Managing expectations is an important part of the medical consultation, therefore enhanced well-being associated with thyrotoxicosis should be part of the physician-patient discussion when treatment with L-T3 is being considered, as well as before introducing treatment for hyperthyroidism.
A major limitation of the hypothesis that enhanced well-being may associated with thyrotoxicosis, is the dearth of studies specifically designed to investigate this putative phenomenon.
5. Conclusions
For some patients, enhanced well-being associated with thyrotoxicosis is probably a feature of overtreatment with thyroid hormones, especially L-T3 containing preparations, and of untreated or partially treated hyperthyroidism. The evidence for enhanced well-being associated with thyrotoxicosis is tenuous and this concept remains a hypothesis. It is an area of thyroidology that is neglected, yet it may explain why L-T3 containing preparations are pursued by some patients as well as why some patients with hyperthyroidism do not adhere to anti-thyroid medication. This is a topic of both academic and clinical importance worthy of further investigation.