1. Background
Thyroid cancer is the most common endocrine malignancy that accounts for 3.4% of all cancers worldwide, and its prevalence has been on the rise worldwide in the last few decades (1). Thyroid cancer in Iran is the seventh most common type of cancer in women, the fourteenth in men, and the eleventh in both sexes. According to the Iranian Cancer Institute, thyroid cancer accounts for 1.8% of all cancers and 76.1% of endocrine cancers (2).
Differentiated thyroid cancers (DTCs), including papillary and follicular carcinomas that originate from epithelial cells, account for the majority (more than 90%) of thyroid malignancies (1, 3). Initial treatment in most patients with DTC includes thyroidectomy and radioactive iodine treatment (4, 5).
Differentiated thyroid cancer has a good prognosis in most patients, and a 10-year survival rate of over 95% has been reported in recent years. However, there is always a risk of recurrence (6, 7). Therefore, it is necessary to evaluate response to treatment in DTC patients in order to prevent over-treatment in low-risk patients. Taking appropriate intervention measures in high-risk patients, on the other hand, is of equal importance. For these reasons, different stratification systems have been developed to assess the response to treatment for this disease (8, 9).
The recurrence risk classification is based on clinical and pathological conditions such as the number of foci of vascular attack, the number and size of lymph nodes involved, the presence of extra-lymph node proliferation, and BRAF/TERT gene mutation (9). After receiving initial treatment, according to this classification, patients diagnosed with DTC are divided into three groups: Low-risk, intermediate-risk, and high-risk (6). However, the risk of actual recurrence in DTC is a dynamic process that changes based on the course of the disease and the response to treatment during follow-up (10-12). Because the initial risk of the tumor alters disease progress or response to treatment, data including serum thyroglobulin (Tg) and anti-Tg levels, as well as structural diagnosis of the disease by imaging during follow-up, are also taken into account when revising the initial risk. This is known as “dynamic risk stratification” (5). The dynamic risk stratification system for DTC patients has been endorsed by the American Thyroid Association (ATA) guidelines and in some other studies (13-15).
2. Objectives
Due to the importance of timely diagnosis of and intervention for DTC, as well as the fact that no study had investigated applying dynamic stratification method to differentiated thyroid cancers in Iran, this study aimed to evaluate using this method to assess response to treatment in DTC patients referring to endocrine clinics in Ahvaz, Iran in 2020 - 2021.
3. Methods
The present study was a cross-sectional, retrospective study that examined the medical records of patients with thyroid cancer who had referred to the endocrine clinics of university hospitals in Ahvaz from April 2020 to May 2021. It was carried out after receiving approval from the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (Ref. ID: IR.AJUMS.HGOLESTAN.REC.1400.020). Moreover, all stages of this study were in accordance with the Declaration of Helsinki, and the principles of patient information confidentiality were strictly observed.
The medical records of patients with DTC were included after surgery. All patients were diagnosed with DTC based on pathological findings of post-thyroidectomy. All decisions regarding the type of surgery and RAI ablation were made in accordance with the 2016 ATA guidelines (13). The inclusion criteria were: (1) adequate clinical and pathological findings to determine the initial risk of recurrence (RR); and (2) follow-up for at least six months after total thyroidectomy with or without lymph node resection ± remnant radioiodine ablation after thyroid hormone withdrawal. The rate of recurrence in patients was classified as low, intermediate, and high risk based on ATA 2016 risk stratification system, which were ≤ 5%, 6 - 20%, and > 20%, respectively. To assess the clinical condition in response to the initial therapy, Tg and thyroglobulin antibody (TgAb) measurements were obtained based on levothyroxine suppression, and neck ultrasonography was performed 6 - 12 months after initial therapy. A stimulated Tg value was also obtained. When a measurable, stimulated, or unstimulated Tg and/or suspicious neck ultrasound findings were observed during a patient follow-up, imaging was then performed. This included computed tomography (CT) or 18-fluorodeoxyglucose positron emission tomography (PET). Suspicious nodes > 10 mm in diameter in ultrasonography were biopsied for cytology. Radioiodine treatment was performed after endogenous TSH (thyroid hormone withdrawal for at least 3 weeks) and using activities of 30 – 100 mCi 131I for low-risk, 100 - 150 mCi 131I for intermediate-risk, and ≥ 150 mCi for high-risk patients. Samples for Tg and TgAb measurement were obtained on the day of ablative radioiodine administration and during follow-up. For TgAb assays, values > 20 Iu/mL were considered to be positive.
3.1. Dynamic Risk Stratification
Patients were stratified into four groups based on the dynamic risk stratification, proposed by the ATA guidelines 2016 (Table 1) (9).
| Total Thyroidectomy + RA | Total Thyroidectomy Without RA a | |
|---|---|---|
| Excellent | Non-stimulated Tg level < 0.2 ng/mL; stimulated Tg level < 1 ng/mL; undetectable anti-Tg; negative imaging | Non-stimulated Tg level < 0.2 ng/mL; undetectable anti-Tg; negative imaging |
| Indeterminate | Non-stimulated Tg level 0.2 - 1 ng/mL; stimulated Tg level 1 - 10 ng/mL; stable or declining TgAb levels; nonspecific findings on imaging studies | Non-stimulated Tg level 0.2 - 5 ng/mL; stable or declining TgAb levels; nonspecific findings on imaging studies |
| Biochemical incomplete | Non-stimulated Tg level < 1 ng/mL; stimulated Tg level >10 ng/mL; increasing anti-Tg levels; negative imaging | Non-stimulated Tg level > 5 ng/mL; increasing anti-Tg levels; negative imaging |
| Structural incomplete | Structural or functional evidence of disease |
Response-to-Therapy Definitions According to the Initial Treatment Performed in Patients with Differentiated Thyroid Cancer
3.2. Statistical Analysis
Using Cochran’s formula and assuming α = 0.05 and P = 0.10 for the rate of distant metastasis in DTC patients, the sample size was calculated to be 154 patients (5).
SPSS version 23 (SPSS Inc., Chicago, IL, U.S.A.) was used for statistical analysis. Mean, median, standard deviation, frequency, and percentage were used to describe the data. Chi-square test or Fisher’s exact test was used to determine the relationship between variables. Significance level in the tests was set at 0.05.
4. Results
The characteristics of patients included in this study are presented in Table 2. All patients had total thyroidectomy. Out of the 154 studied patients, 10 patients had lobectomy before total thyroidectomy, and 37 ones did not have radioiodine ablation (micro-PTC). Majority of the patients had papillary thyroid carcinoma (97.4%). The median age of the patients was 38.5 years (range 15 - 70; mean 40.37 ± 12.34), and the prevalence of DTC in women was four times higher than that in men (81.8% vs. 18.2 %). Furthermore, most patients were in stage I cancer at the time of diagnosis. Response to treatment was assessed during follow-up visits with a mean of 28.59 ± 28.01 months and a median of 18 months in terms of suppressed and/or stimulate Tg values, neck ultrasound, diagnostic whole-body scans, and additional morphological/functional images when considered clinically appropriate.
| Variables | Mean ± Standard Deviation | Median (Range) | Percentage (%) |
|---|---|---|---|
| Age at diagnosis (y) (n = 154) | 40.37 ± 12.34 | 38.5 (15 - 70) | |
| Gender | |||
| Female (n = 126) | 81.8 | ||
| Male (n = 28) | 18.2 | ||
| Histology | |||
| Papillary (n = 150) | 97.4 | ||
| Follicular (n = 4) | 2.6 | ||
| TNM stage | |||
| Stage I (n = 149) | 96.75 | ||
| Stage II (n = 5) | 3.25 | ||
| Follow-up duration, months (n = 154) | 28.59 ± 28.01 | 18 (6 - 120) | |
| 131I cumulative dose, mCi (n = 117) | 147.05 ± 85.50 | 150 (30 - 595) | |
| Risk of recurrence (modified 009 RRS) | |||
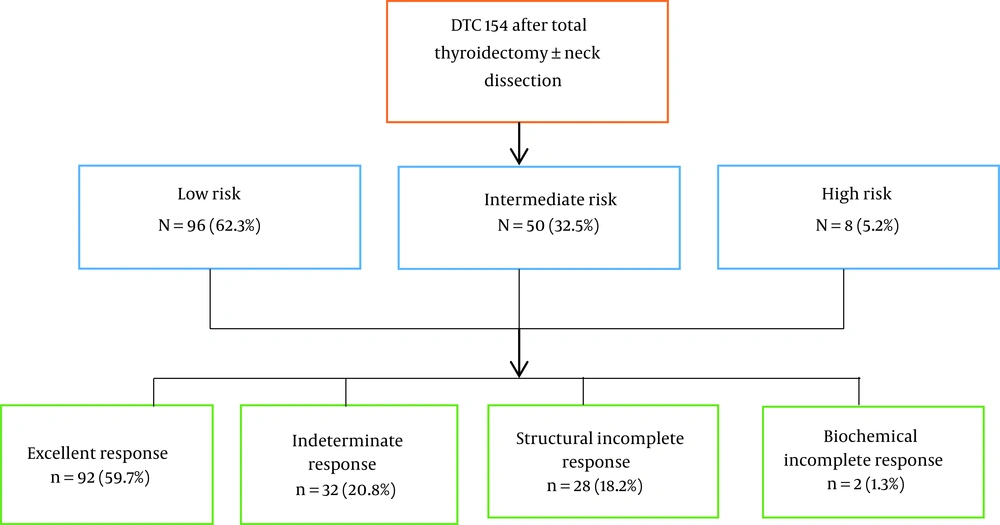
| Low risk (n = 96) | 62.3 | ||
| Intermediate risk (n = 50) | 32.5 | ||
| High risk (n = 8) | 5.2 | ||
| BMI (17.96 - 44.14) | |||
| < 25 (n = 47) | 30.5 | ||
| 25 - 29.99 (n = 71) | 46.1 | ||
| > 30 (n = 36) | 23.4 | ||
| History of diabetes (n = 14) | 9.01 | ||
| Family history of thyroid cancer (n = 27) | 17.53 |
Basic Characteristics of 154 Patients with Differentiated Thyroid Cancer
Assessing the risk of primary recurrence revealed that 96 (62.3%), 50 (32.5%), and 8 (5.2%) patients were in low, intermediate, and high risk of recurrence, respectively. Based on the dynamic stratification system, excellent response in 92 patients (59.7%), indeterminate response in 32 patients (20.8%), biochemical incomplete response in 2 patients (1.3%), and structural incomplete response in 28 patients (18.2%) were observed.
The results of response to treatment of differentiated thyroid cancers at the end of follow-up in all three risk groups are presented in Table 3. There were significant differences among the risk groups in terms of response to treatment (P < 0.001). Comparison of results of response to treatment in the three categories of risk recurrence based on dynamic stratification is shown in Table 4. As can be seen in Table 4, ER in the low-risk group was significantly higher than that in other groups (79.2% vs. 32%; P < 0.0001). Moreover, 32% of the patients classified as having intermediate RR had an excellent initial response to treatment (P < 0.0001). No ER was observed in the high-risk group, while this rate in the other groups was 59.7% (P = 0.001).
| Response to Treatment | Low Risk (n = 96) | Intermediate Risk (n = 50) | High Risk (n = 8) | Total |
|---|---|---|---|---|
| ER | 76 (79.2) | 16 (32) | 0 (0) | 92 (59.7) |
| IR | 15 (15.6) | 17 (34) | 0 (0) | 32 (20.8) |
| SIR | 5 (5.2) | 17 (34) | 6 (75) | 28 (18.2) |
| BIR | 0 (0) | 0 (0) | 2 (25) | 2 (1.3) |
Frequency of Initial Response to Treatment of Differentiated Thyroid Cancers in the 3 Groups of Risk of Recurrence a
5. Discussion
In this cross-sectional and retrospective study, 154 patients with DTC and with a follow-up median of 18 months (range 6 - 120; mean 28.59 ± 28.01) were examined. It was found that out of all patients classified as having low RR, 79.2% had an excellent response after initial therapy, and a minority of the low-risk patients (5.2%) had SIR. Patients classified as having intermediate RR were less likely to have an excellent response to treatment, and patients with an initial high RR showed no excellent response to treatment, while this rate was 59.7% in other groups. Taking into account these results, it was argued that the dynamic risk stratification method may have been used in the management and treatment of differentiated thyroid cancers in the study population.
Tuttle et al. conducted a retrospective study on 588 patients with DTC undergoing total thyroidectomy and radioactive iodine treatment during an average 7-year follow-up period (16). Among patients who had an excellent response to initial treatment, the probability of having a structural incomplete response fell from 3% to 2% in low-risk patients, from 21% to 2 % in patients with intermediate risk, and from 68% to 14% in those in the high-risk group. Conversely, structural or biochemical incomplete response increased the percentage of stable structural disease at the end of follow-up to 13%, 41%, and 79% in low, intermediate, and high-risk patients, respectively (16). Comparing two methods of static (initial recurrence risk) and dynamic stratification showed that when the recurrence risk was taken into account, the proportion of the variance explained (PVE) was 34%. Proportion of the variance explained measures the ability of a stratification system to predict the final response (structural incomplete response versus excellent response). When response to treatment was taken into account during the first two years after initial treatment, however, PVE rose to 84% (16). In another retrospective cohort study by Castagna et al., the percentage of patients achieving complete recovery at the end of follow-up was 90% for patients who initially had a low risk, whereas it was 96% for patients who had an excellent initial response to treatment (17). Importantly, the percentage of complete recovery in patients with high dynamic risk (i.e., patients with initial biochemical persistence or incomplete structural response) decreased from 60% to 27%. The positive predictive value for dynamic risk stratification was 72.8%, and that for recurrence risk stratification was 39.2%, which means that dynamic risk assessment has a greater ability to predict the response to treatment (17).
The results of the study by Mukhtar et al. demonstrated that the response to treatment varied based on dynamic risk stratification during follow-up (10). In their study, excellent response to treatment increased from 52.5% in the initial evaluation to 83.2% in the last visit (mean follow-up of 105 months), and decreased from 25% to 1% in the last visit in the indeterminate response group. The two groups of biochemical incomplete response (6.4% to 3.6%) and structural incomplete response (16.2% to 12.2%) underwent fewer changes. Therefore, given the fact that the disease status may change over time and that the risk is a dynamic phenomenon which should be assessed at each visit (9, 11, 13, 16), the dynamic risk assessment system is a perfect tool for assessing response to treatment in DTC patients, whose positive predictive value has been reported in previous studies (9-12, 18, 19).
According to our study results, most patients with excellent response to treatment were in the low-risk group and had a good prognosis. On the other hand, patients with structural disease needed more monitoring and intervention since a significant number of these patients were in the high-risk group, which was consistent with previous studies (9, 10). In Momesso et al., for instance, during a two-year follow-up after lobectomy treatment, none of the patients with excellent response to treatment experienced recurrence of the disease, and recurrence was only observed in 4.3% of the patients in the IR group, and 33.3% of the patients in the BIR group (18). Also, in patients undergoing total thyroidectomy, while no recurrence was observed in ER, IR, and BIR groups, all six patients with structural incomplete response experienced recurrence in the two-year follow-up (18).
Good prognosis in patients with ER has also been reported in other studies (10, 20-22). In a study by Hong et al., 60.2% of intermediate-risk patients and 20.5% of high-risk patients were stratified in the excellent response group (20). In Jeon et al., out of all DTC patients with excellent response to initial treatment during a follow-up period of 7.8 years, only 1% had a recurrence of the disease (22).
At an average follow-up period of 92.8 months, Pitoia et al. reported excellent response to treatment in 74.7% of the low-risk group, 45.3% of the intermediate-risk group, and 10.3% of the high-risk group (23). In a review of several articles by Pitoia and Jerkovich, moreover, excellent response to treatment was usually observed in 86 - 91% of patients with low risk of recurrence in the final follow-up, and the rates of excellent response to treatment in the intermediate- and high-risk groups were 57 - 63% and 14 - 16%, respectively (9).
According to Dehbi et al., the recurrence rate in patients with differentiated thyroid cancer in the excellent response to treatment group based on dynamic stratification for an average follow-up period of 6.5 years was 0% (4) while, according to Schlumberger et al., the same rate was 0.16% for an average follow-up period of 5.4 (24). These results were in line with our study findings. In our study, no high risk of recurrence was observed in any of the patients with excellent response to initial treatment. In the study by ven Velsen et al., on the other hand, during an average follow-up period of 6 years, only 14% of the patients with differentiated thyroid carcinoma who were placed in the excellent response to initial treatment group experienced recurrence based on the dynamic stratification (25). In Tian et al., moreover, the recurrence rate in patients with differentiated high-risk thyroid cancer for a follow-up period between 9 - 12 months after initial treatment was reported to be 2.9% in the excellent response to treatment group (26). Therefore, physicians should be aware of the risk of disease recurrence in this group of patients, even after their excellent response to initial treatment.
The discrepancies observed between our study results and those of other studies may have been due to the smaller number of patients in the high-risk group and the shorter length of the follow-up period in our study compared to those in similar studies, resulting in a higher percentage of patients in the IR group. Due to the lack of appropriate diagnostic facilities in the present study, moreover, it was not possible to investigate the mutations which may have led to problems in the initial stratification of patients.
The use of a dynamic risk assessment system in DTC patients has been confirmed in other studies. Park et al., for example, found that during a follow-up period of 8.6 years, the risk of disease recurrence in DTC patients was significantly different based on response to treatment, and hazard ratios (HR) of disease recurrence in the IR, BIR, and SIR groups were 1.82, 20.8, and 243.3, respectively (14). The rate of excellent response to treatment in the low-risk of recurrence group was reported to be 72.7%, while no excellent response was observed in the high-risk group (14). In Abelleira et al., the results of a dynamic risk assessment during a postoperative follow-up of DTC patients for at least 12 months showed no recurrence in any of the patients with excellent response to treatment. In patients with indeterminate initial response at the end of the follow-up period, however, disease progress was observed in 33.4% of the cases (27). These results were consistent with the findings of the present study, suggesting that the risk of recurrence varied based on response to treatment during follow-up.
Over the past decade, recognition of the patterns and clinical course of DTC has led to significant changes in disease management based on assessment and estimation of the risk of recurrence and mortality (11). Low-risk DTC patients usually undergo less invasive therapies such as pharmaceutical therapies or lobectomy, whereas in high-risk patients, invasive treatments including thyroidectomy and adjuvant treatment with radioactive iodine, and follow-up at shorter intervals are taken into account from the very outset (9, 11, 13).
Risk assessment and response to treatment in the follow-up period after initial treatment based on information available at the time of assessment has been developed as “dynamic risk assessment” (13, 16, 18). Therefore, using dynamic stratification for assessing response to treatment in patients with DTC alters the management of thyroid cancer from an already specified and inflexible management approach adopted for all patients to an individualized model of management and treatment that involves recurrence risk assessment based on the data obtained during the follow-up period for every single patient (10, 16). Thus, it can be used to prevent over-treatment and additional follow-up in patients with good prognosis, and to offer appropriate treatment measures for patients with poor prognosis. Since the mortality rate in DTC is very low, most specialists have changed their diagnostic and treatment methods for the low-risk group (10-12).
The present study had several limitations including a shorter follow-up of patients compared with other studies, which resulted in larger frequency of indeterminate response; these patients may have been eventually reclassified in any of the categories in longer follow-ups. Other limitations were the failure to investigate mutations such as BRAF and TERT in patients as well as the small sample size. Therefore, it was recommended that future studies should be carried out by employing larger sample sizes and longer follow-up periods as well as performing biological examination of the samples in a multi-center setting. Such measures may have facilitated obtaining more reliable results and, thus, developing a more accurate treatment plan.
5.1. Conclusions
It was concluded that the response to initial treatment significantly varied based on dynamic risk stratification, with ER being highest in the low-risk group compared to the other groups, and that a minority of the low-risk patients (%5.2) had SIR at evaluation. Patients classified as having intermediate RR were less likely to have an excellent initial response to treatment, and no ER was observed in the high-risk group. To evaluate the response to treatment based on the new system of dynamic risk assessment in DTC patients undergoing thyroidectomy and radioiodine treatment, therefore, our study results may have been used in the clinical setting for predicting the response to treatment and determining the treatment plan in study population more accurately (Figure 1).