1. Background
Congenital hypothyroidism (CH) (i.e., thyroid hormone deficit at birth) is a common endocrine disorder with a 1: 2000 to 1: 4000 incidence rate in neonates. It is one of the most prevalent preventable causes of physical and mental impairment worldwide (1). Congenital hypothyroidism is associated with problems in thyroid gland dysgenesis or thyroid hormone synthesis during embryonic development. Numerous abnormalities in thyroid developing genes and environmental factors such as iodine deficit or excess have been linked to an increased risk of CH (2-5).
In the 1970s, a worldwide newborn screening program (NBS) was launched with remarkable effectiveness in reducing CH-related problems (6). Prior to the development of the NBS, primary CH was primarily diagnosed using a 12-item checklist (persistent jaundice, constipation, umbilical hernia, poor weight gain, xerosis cutis, sluggishness, macroglossia, hoarseness, coldness of limbs, edema, dilation of posterior fontanel, and goiter) (7). Many neonates with CH, on the other hand, appear normal. At birth, clinical signs are mild and non-specific. Since less than 5% of babies exhibit clinical signs, they are frequently diagnosed late or missed (8).
In 1972, research on hypothyroidism screening began in North America (9). Ordookhani et al. initiated hypothyroidism screening programs in Iran in 1987 (10). It is noteworthy that the disease is twice as prevalent in girls than in boys (11). Congenital hypothyroidism incidence varies in different countries (12-15). Within North America, geographic variations in CH incidence were also found. A study conducted in the United States of America discovered a higher prevalence in the southwest, the Great Lakes, and Hawaii (16). The trend of disease in a particular region varies over time (12, 14, 17). Iran has a higher prevalence of CH than other countries in the world (13, 15, 17). Previous studies have shown that the prevalence of CH in Asian countries, particularly in Iran, is higher than the world average because of iodine deficiency, consanguineal marriage, and genetics (18). The incidence of the disease in different areas of Iran varies from 1: 400 to 1: 1000 (18, 19).
The Iranian national neonatal screening program for diagnosis and treatment of children with CH, as a national strategy, was established between 2004 - 2005 (20). Available data show that the physical growth of CH cases has remained within normal age and gender and matched the healthy children. Infants with CH have an excellent prognosis if diagnosed early and treated promptly (21, 22). Hence, proper evaluation of the program’s impact using valid instruments can help policymakers and health providers improve CH surveillance in Iran.
2. Objectives
This study mainly aimed to examine the physical growth of hypothyroid children diagnosed by the national CH screening program at the age of six in order for evaluating the success of early treatment. It also aimed to compare the anthropometric indexes of children with CH taking levothyroxine with those of children without CH in order for assessing the efficiency of the CH screening program.
3. Methods
3.1. Study Design and Population
The present research was a retrospective cohort study carried out in five provinces in Iran. The study population was 480 children divided into two groups including 240 (50%) children with CH and the remaining 240 (50%) healthy children. The anthropometric indexes, including weight, height, and head circumference, were measured for the two studied groups of children aged six in 2015 and 2016. Children with CH had been diagnosed and treated with levothyroxine by the national newborn screening program six years before the study in 2009. The CH children consisted of transient and permanent cases. Permanent CH refers to a persistent deficiency of thyroid hormone that requires life-long treatment. Transient CH refers to a temporary deficiency of thyroid hormone discovered at birth but then recovered to normal thyroid hormone production, with no need for treatment after the age of three. The samples were taken using systematic random sampling from a dataset derived from Iran’s national newborn screening registry. For every case, one healthy child as control was matched with a child with CH based on age, sex, and residence location. For age-matching, the control selected for each case was a child born within ±3 months from the CH child’s birthdate. A serious attempt was made to assign the children with similar socioeconomic status to two groups and, therefore, the children in the control group were randomly selected from among the children born in the same areas (i.e., the same neighborhood or village).
3.2. Data Collection
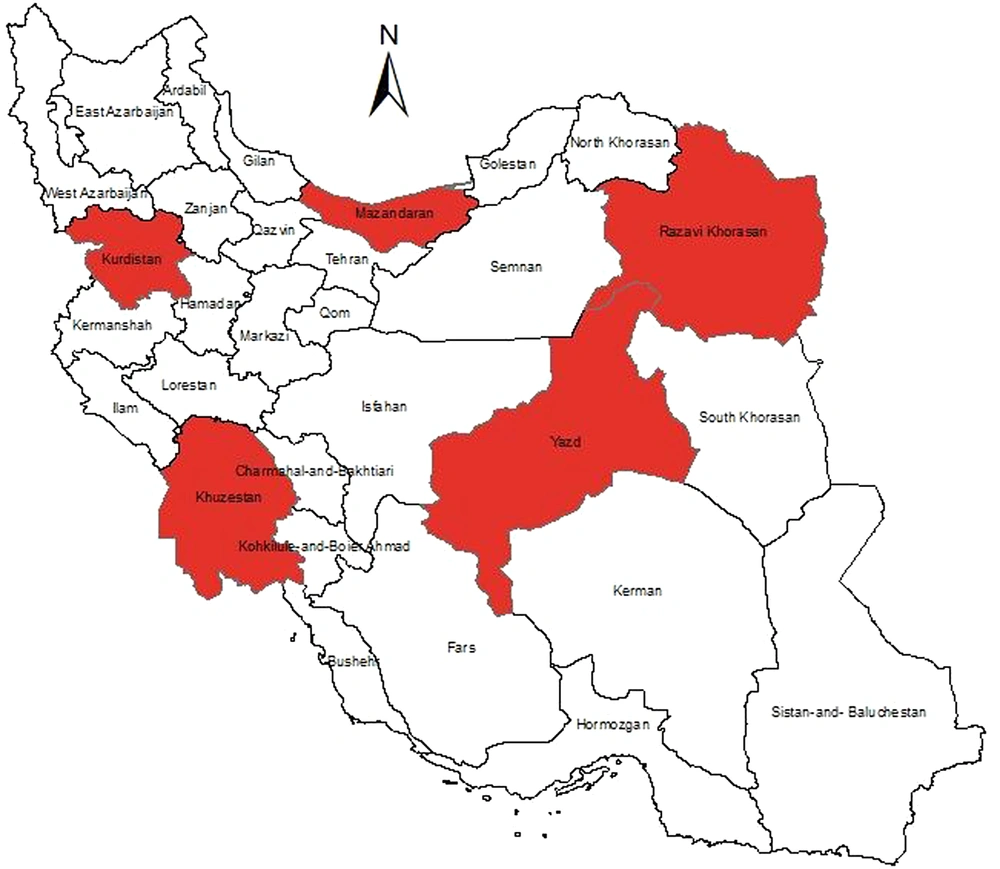
Given the importance of the results’ generalizability, the country was initially divided into five regions: North, south, east, west, and center. Therefore, Mazandaran, Khuzestan, Khorasan Razavi, Kurdistan, and Yazd provinces were randomly selected from each region (Figure 1). The sample population was determined for each province using either the diagnosed CH population or the probability proportional to size (PPS) sampling approach.
Concurrent disease, malnutrition, intrauterine growth restriction, small fetus size for gestational age, and children with genetic abnormalities such as down syndrome or severe deformities were excluded. In each of the five provinces, sampling was conducted using the systematic sampling method after obtaining written consent from parents, ensuring confidentiality, avoiding imposed costs, and adhering to required endorsements by the provincial health center and data on the anthropometric indexes of children aged birth to six years. Height (cm), weight (g), and head circumference (cm) of children under the age of two were measured. The measuring scale used in all provinces was the same. OMRON scale (a confirmed tool by WHO) was our tool to obtain the values of weight and height. The mean, median, and standard deviation were calculated for the treatment and control groups based on their age and gender and, then, they were compared for data description.
3.3. Ethical Consideration
This study was performed in accordance with the Declaration of Helsinki. Accordingly, written informed consent was obtained from the children’s parents before the data collection. In addition to obtaining informed written consent, an incentive was also given to the children for participating in the study. Additionally, the participants were allowed to withdraw from the research at any moment without jeopardizing their child’s treatment plan.
3.4. Statistical Analysis
All analyses were conducted using t-test and two-way ANOVA statistics performed by STATA software version 14. Although ANOVA is considered a robust test against the normality assumption, violation assumptions were assessed using the Shapiro-Wilk test. Another assumption of ANOVA, homogeneity of variances, was also checked by Levine’s test, and our data satisfied this assumption. The two main assumptions of the independent-samples t-test, including normality of anthropometric indexes as dependent variable and homogeneity of variances using the Kolmogorov-Smirnov test and Levine’s test, were also assessed.
4. Results
In this study, 480 children were divided in two groups, namely the CH group with 240 samples and the healthy (control) group with 240 samples. A total of 131 (54.6%) of the CH samples had transient congenital hypothyroidism (TCH), whereas 109 (45.4%) had permanent congenital hypothyroidism (PCH). Some of the demographics of the studied population are presented in Table 1.
| Characteristic | Congenital Hypothyroidism Cases (n = 240) | Control Group (n = 240) | Total |
|---|---|---|---|
| Sex | |||
| Male | 118 (49.2) | 118 (49.2) | 236 (49.2) |
| Female | 122 (50.8) | 122 (50.8) | 244 (50.8) |
| Province | |||
| Khuzestan (located in southwest of Iran) | 69 (28.7) | 69 (28.7) | 138 (28.7) |
| Khorasan Razavi (located in northeast of Iran) | 51 (21.2) | 51 (21.2) | 102 (21.2) |
| Kurdistan (located in west of Iran) | 50 (20.8) | 50 (20.8) | 100 (20.8) |
| Yazd (located in centre of Iran) | 40 (16.6) | 40 (16.6) | 80 (16.6) |
| Mazandaran (located in north of Iran) | 30 (12.5) | 30 (12.5) | 60 (12.5) |
| Gestational age | |||
| Pre-term (< 37weeks) | 24 (10) | 17 (7.1) | 41 (8.5) |
| Term (37 - 42 weeks) | 209 (87.1) | 219 (91.2) | 428 (89.2) |
| Post-term (> 42 weeks) | 7 (2.9) | 4 (1.7) | 11 (2.3) |
| Age (y) b | 6 ± 0.4 | 6 ± 0.3 | 6 ± 0.35 |
| Weight at birth (g) | 3198 ± 605 | 3294 ± 553 | 3246 ± 569 |
a Values are expressed as No. (%) or mean ± standard deviation.
b Age: Age at the time of conducting the study
As shown in Table 1, the population (%) of children with CH born out of term was higher than the healthy (control) group, and this difference in baseline was statistically significant (P < 0.001). The number of CH cases and their controls were equal by gender and province variables. There was no statistical difference between two study groups in terms of the mean birth weight (P = 0.07).
As presented in Table 2, the mean weight, height, and head circumference of CH children aged six were 20304.8 (95% CI: 19732.9 - 20873.3) g, 115.6 (95% CI: 114.8 - 116.3) cm, and 50.8 (95% CI: 50.6 - 51.0) cm, respectively. The mean of these measures in healthy children was 20741.2 (95% CI: 20186.0 - 21291.6) g, 116.7 (95% CI: 115.9 - 117.5) cm, and 51.1 (95% CI: 50.9 - 51.3) cm, respectively.
| Province of Residence | Weight (g) | Height (cm) | Head Circumference (cm) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Congenital Hypothyroidism Cases | Controls | P-Value | Congenital Hypothyroidism Cases | Controls | P-Value | Congenital Hypothyroidism Cases | Controls | P-Value | |
| Khuzestan | 20712 ± 4921 | 20749 ± 4677 | 0.5 | 115.8 ± 6.1 | 116.8 ± 5.9 | 0.3 | 50.7 ± 1.2 | 51.2 ± 2.0 | 0.1 |
| Khorasan Razavi | 19760 ± 3422 | 20749 ± 4677 | 0.2 | 113.7 ± 5.4 | 115.0 ± 6.1 | 0.2 | 50.7 ± 1.5 | 50.9 ± 1.5 | 0.5 |
| Kurdistan | 20422 ± 4571 | 19604 ± 2695 | 0.3 | 117.4 ± 6.1 | 117.8 ± 4.4 | 0.7 | 50.9 ± 1.5 | 50.8 ± 1.1 | 0.9 |
| Yazd | 20052 ± 4477 | 20222 ± 4259 | 0.9 | 115.3 ± 5.1 | 116.4 ± 7.9 | < 0.5 | 51.2 ± 1.5 | 51.1 ± 1.2 | 0.6 |
| Mazandaran | 20433 ± 4845 | 22210 ± 5304 | 0.2 | 115.5 ± 6.1 | 117.9 ± 6.1 | 0.1 | 50.6 ± 1.9 | 52.0 ± 2.4 | 0.01 |
| P value | < 0.8 | < 0.08 | 0.03 | 0.1 | 0.5 | 0.03 | |||
| Total | 20304 ± 4457 | 20741 ± 4337 | 0.3 | 115.6 ± 5.9 | 116.7 ± 6.1 | 0.04 | 50.8 ± 1.7 | 51.1 ± 1.7 | 0.03 |
The mean ± standard deviation (SD) of weight in the control group (20741.2 ± 4337.3 g) was higher than that in the CH group (20304.8 ± 4457.9 g), but the difference was not statistically significant (P = 0.3). The mean difference of weight between the five studied provinces was not statistically significant (CH (P = 0.8), control (P = 0.08)). It is noteworthy that the difference between the case and control groups regarding the weight component was not significant in any of the studied provinces.
Comparison of height measurements revealed that the mean ± SD of height in the control group (116.7 ± 6.1 cm) was significantly higher than that in the CH group (115.6 ± 5.9 cm) (P = 0.04). According to Table 2, although this difference was generally significant, the differences between the CH and healthy children were not significant individually in any of the provinces.
Mean ± SD of head circumference for CH cases and healthy children (control group) were 50.8 ± 1.7 cm and 51.1 ± 1.7 cm, respectively. The difference between two studied groups regarding the mean of head circumference was statistically significant (P = 0.03). The obtained data indicated that this difference between the CH and healthy children from all provinces was insignificant, except for those from Mazandaran (P = 0.01). No significant difference was observed between two groups in subgroup analysis in terms of the mean of physical development indexes, based on the type of CH and gender, except for the mean of head circumference (Table 3); therefore, the mean of head circumferences in TCH patients (50.6 ± 1.4 cm) was significantly lower than that in healthy children (51.1 ± 1.7cm) (P = 0.004).
| Variables | Weight (g) | Height (cm) | Head Circumference (cm) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Congenital Hypothyroidism Cases | Controls | P-Value | Congenital Hypothyroidism Cases | Controls | P-Value | Congenital Hypothyroidism Cases | Controls | P-Value | |
| Congenital hypothyroidism type | |||||||||
| Transient congenital hypothyroidism | 20119 ± 3971 | 20715 ± 4293 | 0.2 | 115.7 ± 5.6 | 116.9 ± 6.7 | 0.1 | 50.6 ± 1.4 | 51.1 ± 1.7 | 0.004 |
| Permanent congenital hypothyroidism | 20527 ± 4990 | 20772 ± 4409 | 0.7 | 115.4 ± 6.2 | 116.5 ± 5.4 | 0.1 | 51.0 ± 1.9 | 51.2 ± 1.7 | 0.3 |
| Gender | |||||||||
| Boy | 21006 ± 4848 | 21478 ± 4282 | 0.4 | 116.4 ± 5.5 | 117.7 ± 6.1 | 0.09 | 51.1 ± 1.7 | 51.4 ± 1.7 | 0.09 |
| Girl | 19625 ± 3946 | 20028 ± 4278 | 0.4 | 114.7 ± 6.1 | 115.7 ± 5.0 | 0.2 | 50.6 ± 1.6 | 50.9 ± 1.7 | 0.2 |
5. Discussion
This study was designed to assess the physical growth indexes, including weight, height, and head circumference, of CH cases at the age of six and compare them with those of healthy children as the control group. After adjusting for age, gender, and socioeconomic level (SES), the researchers discovered that the weight, height, and head circumference of CH and healthy children were approximately comparable, with no significant difference between the two groups in all studied provinces. The findings underscored the essential nature and significance of early detection and treatment of children with CH in the research area.
Numerous studies have revealed that children with CH experience growth restriction (23-25). Previous studies have also indicated that physical growth failure could be avoided by early detection and treatment (26, 27). Moschini et al. determined that the children’s normal height growth was observed at the age of six, and the mean age for administering the treatment was at 33 days of age (24). The finding of a Chinese study comparing the height and weight of CH children with those of healthy children aged 0 (birth) to 12 years showed that height growth was similar in the two groups (28). In an Iranian study on children aged seven, the mean height of CH children was considerably lower than that of healthy children; however, no significant difference in mean weight was detected between the two groups (20). According to the guidelines of the neonatal hypothyroidism, the most important points in preventing the developmental defects of the affected children are early detection and timely treatment. In a longitudinal study by Heidari et al., it was shown that the appropriate dose at the beginning of treatment, the time of diagnosis, and the time of treatment initiation were the most important predictors of children’s growth and development (29). Although Iran, at the time of the study, had low cases of delayed diagnosis due to the implementation of the newborn screening, the delay in diagnosis and treatment was one of the problems of developing countries (30).
The results of this study demonstrated that newborns with CH had a slightly larger mean head circumference than healthy infants, though the difference was not significant (31). A study in Sweden revealed that the average weight in the first three years of life in CH children diagnosed and treated in time was similar to that in the control group (32). According to a study conducted in Japan on 2,341 children with CH, both CH and healthy children had normal height growth (33). Lotfi et al. found no significant difference between the growth patterns of CH and healthy children after five years of age in terms of height, weight, and head circumference (34).
In sum, our data demonstrated the efficiency of Iran’s National Newborn Screening Program in detecting and treating CH cases promptly as well as in eradicating severe physical growth limits caused by CH. Nonetheless, hypothyroid children were found to have lower anthropometric indexes than healthy children. Therefore, it was suggested that an increased emphasis should be placed on surveillance system enhancements, particularly on regular clinical examination and systematic monitoring of serum thyroid hormone levels in children with CH, in order to ensure adequate thyroxin compensation. It was also highly recommended that children with CH should undergo continuous surveillance for the first six years of their lives, particularly in cases of PCH., since this may have assisted the health policymakers, health practitioners, and parents of CH children to manage congenital hypothyroidism and avoid its severe negative effects.
The strength of this study lies in the fact that it adopted a historical cohort design by using the national data and comparable controls as well as the large samples with minimal attrition, and by investigating a population from an expansive geographical area. This study was one of the few studies conducted in the field of physical development exploring the main outcome of CH treatment in Iran, while most previous studies had focused on IQ, age at onset of treatment, and care coverage of CH children (35-37). Although a subgroup analysis was used based on CH type (transient/permanent) and the anthropometric indexes were compared in our study, the main limitation of our study was not the lack of further information about athyreosis and ectopic cases or the lack of TSH and T4 serum levels1 at age of six required for making more appropriate inference. In fact, insufficient information and limited data were the main limitations for performing modeling and interpreting the results based on the adjustment of variables. Another limitation of the present study was the almost equal number of girls and boys in the study groups, whereas the female/male proportion was approximately 60/40 in Iran.
5.1. Conclusions
Although the mean of anthropometric indexes in CH children was slightly lower than that in healthy children aged six, the difference between the two groups was insignificant, and the children with CH were found to enjoy from a good physical development. Our results highlighted the impact and importance of the prompt initiation of LT4 treatment and regular follow-up by using laboratory tests, performing dose adjustments to maintain TH levels within target ranges, conducting appropriate assessment of the treatment duration and necessary continuation, considering the developmental factors, and educating the children and their family about CH.
In conclusion, it was determined that the national newborn screening program to identify children with CH and treat them with levothyroxine resulted in the normal physical growth of children with CH in Iran. It was recommended that further studies and modeling should be conducted in order to assess the role of potential factors contributing to the physical development such as the type of CH, age of treatment, dosage of treatment, and the severity of the disorder.