1. Context
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) induced coronavirus disease 2019 (COVID-19) is still a major global health issue. Globally, more than 6.8 million fatalities and over 760 million confirmed cases have been documented as of March 16, 2023 (1). Diabetes is a frequent comorbidity in people with COVID-19, with a prevalence ranging from 7 to 30% (2). Diabetes is a well-established risk factor for severe disease and poor outcomes in COVID-19 (3). Diabetes-related uncontrolled hyperglycemia is linked to poor innate and adaptive immune responses, which raises the risk of developing severe COVID-19 and its complications (4). New-onset diabetes and worsening hyperglycemia in pre-existing diabetes have also been reported after COVID-19 infection (5).
This bidirectional relationship between COVID-19 and diabetes poses an unprecedented challenge in identifying effective drugs for comprehensive management. Metformin, a widely used drug to treat type 2 diabetes mellitus, was first developed as an anti-influenza agent, and its ability to lower blood glucose was only incidentally observed (6). Metformin is being increasingly studied in SARS-CoV-2 infection for its broad plethora of effects beyond glycemic control.
Metformin has also been preferentially used in patients with prediabetes based on the evidence supported by several diabetes prevention trials. Furthermore, the severity of COVID-19 has been documented to be high even among individuals with prediabetes, further justifying the use of metformin in this setting (7).
2. Objectives
This article reviews the proposed pleiotropic effects of metformin on the various steps of pathogenesis of SARS-CoV-2 infection and the impact of its use on clinical outcomes in COVID-19.
3. Methods
This narrative review aims to describe the role of metformin beyond glycemic control in COVID-19. Web of Science, Google Scholar, Pubmed, and Scopus electronic databases were searched for studies (all year) assessing the role of metformin in COVID-19, using the following text words: "Metformin" and "COVID-19". To identify studies the automated search may have missed, we manually scanned review articles and checked the reference lists of original papers. All relevant experimental, observational, and review studies investigating the role of metformin in COVID-19 were included. We excluded commentaries, case reports, editorials, letters, and studies that did not report accurate and clear data or methods.
4. Results and Discussion
4.1. Pathophysiology of COVID-19 Infection in Diabetes
Diabetes can predispose to severe COVID-19 infection by several distinct mechanisms. These involve a complex interaction between the host's innate and adaptive immune systems (4). Chronic hyperglycemia adversely affects pathogen elimination by polymorphonuclear leucocytes. Diabetes can cause C4 complement deficiency and reduce complement activation, affecting the humoral component of innate immunity. Antibody and cell-mediated adaptive immune responses, carried out by B and T cells, respectively, are also affected by chronic hyperglycemia (8). Diabetes is a proinflammatory state that favors the development of an exaggerated inflammatory response leading to a cytokine storm, a hallmark of severe COVID‐19. A critical mechanism in developing cytokine storms is the chronically active proinflammatory NF-kappa-B (NF-B) pathway found in individuals with diabetes (9). Expression of the angiotensin-converting enzyme 2 (ACE2) receptor, the primary portal to virus entry, is increased in diabetes. Enhanced ACE2 expression in the lungs in diabetes may amplify the viral load and increase the risk of developing severe infections (10). Furthermore, complications of diabetes, such as cardiovascular and chronic kidney disease, could be additional factors complicating recovery (11).
4.2. Metformin in COVID-19: Clinical Evidence
Several clinical studies, mostly retrospective, have found favorable outcomes in COVID-19 in those who received metformin for glycemic control. A sub-analysis of the TARGET-COVID study found that metformin use was associated with lower markers of inflammation, reduced renal ischemia, thrombosis, decrease in hospitalization duration, and requirement for intubation (12). A retrospective cohort study evaluating pre-admission metformin usage in 586 patients found that the metformin group had a 40% reduction in all-cause mortality after hospitalization. Interestingly, this effect started in the in-hospital period but also became significant when evaluated within the post-discharge period of 157.5 (105 - 235.7) [median (IQR)] days (13).
In a retrospective population-based study, a significantly decreased risk of total mortality was linked to metformin. (OR 0.79; 95% CI 0.73 - 0.86) (14). According to the CORONADO trial, only metformin treatment reduced mortality rates compared to all other antidiabetic agents (15). In a study of 64,852 veterans with COVID-19 and diabetes, the total mortality risk was significantly reduced by metformin [OR 0.84 (95% CI 0.78 - 0.91)] (16). Luk et al. reported a decreased incidence of the composite outcome of intensive care unit admission, the need for invasive mechanical ventilation, and in-hospital death (0.51 (95% CI 0.34 to 0.77) with metformin (17). Tamura et al., in a retrospective study of 188 patients, found that the in-hospital non-metformin group had increased mortality (20.5%) compared to the metformin group (3.5%) (P = 0.0002). Patients receiving metformin before hospital admission showed lower severity parameters at admission (18).
A meta-analysis of 2,916,231 patients from 32 cohort studies concluded that metformin was linked to significantly lower mortality rates with an OR of 0.78 [95% CI: 0.69 - 0.88] (19). A Bayesian network meta-analysis of 35 papers examining the correlation between antidiabetic drugs and clinical outcomes reported that metformin users had a significantly reduced mortality rate than non-users. (Pooled OR, 0.74; 95% CI: 0.67 - 0.81; P = 0.00). Bayesian analysis used ranking probabilities to compare the overall impact of each antidiabetic medicine on mortality risk. Metformin had the third and fourth-rank probability of reducing mortality by 44.8% and 38.9%, respectively, after GLP-1 receptor agonists and SGLT2 inhibitors (20).
However, the results conflicted in TOGETHER, a randomized controlled trial (RCT). Treatment with metformin 750 mg twice daily, compared to a placebo for ten days, did not reduce the requirement for hospitalization from worsening disease or offer any therapeutic benefit to ambulatory patients. It was postulated that longer-term use of metformin might provide more significant benefits than acute administration (21). In another RCT involving adults overweight and obese, metformin at 1500 mg/day for 14 days did not prevent a primary event of emergency department visit, hypoxemia, hospitalization, or death. However, the analysis of the prespecified secondary outcome, a composite end-point of hospitalization, emergency department visit, or death, favored metformin use (22).
4.3. The Pathophysiologic Link Between Metformin and COVID-19 Infection
As summarized below, metformin can be protective in various stages of COVID-19 pathogenesis (Figures 1 and 2).
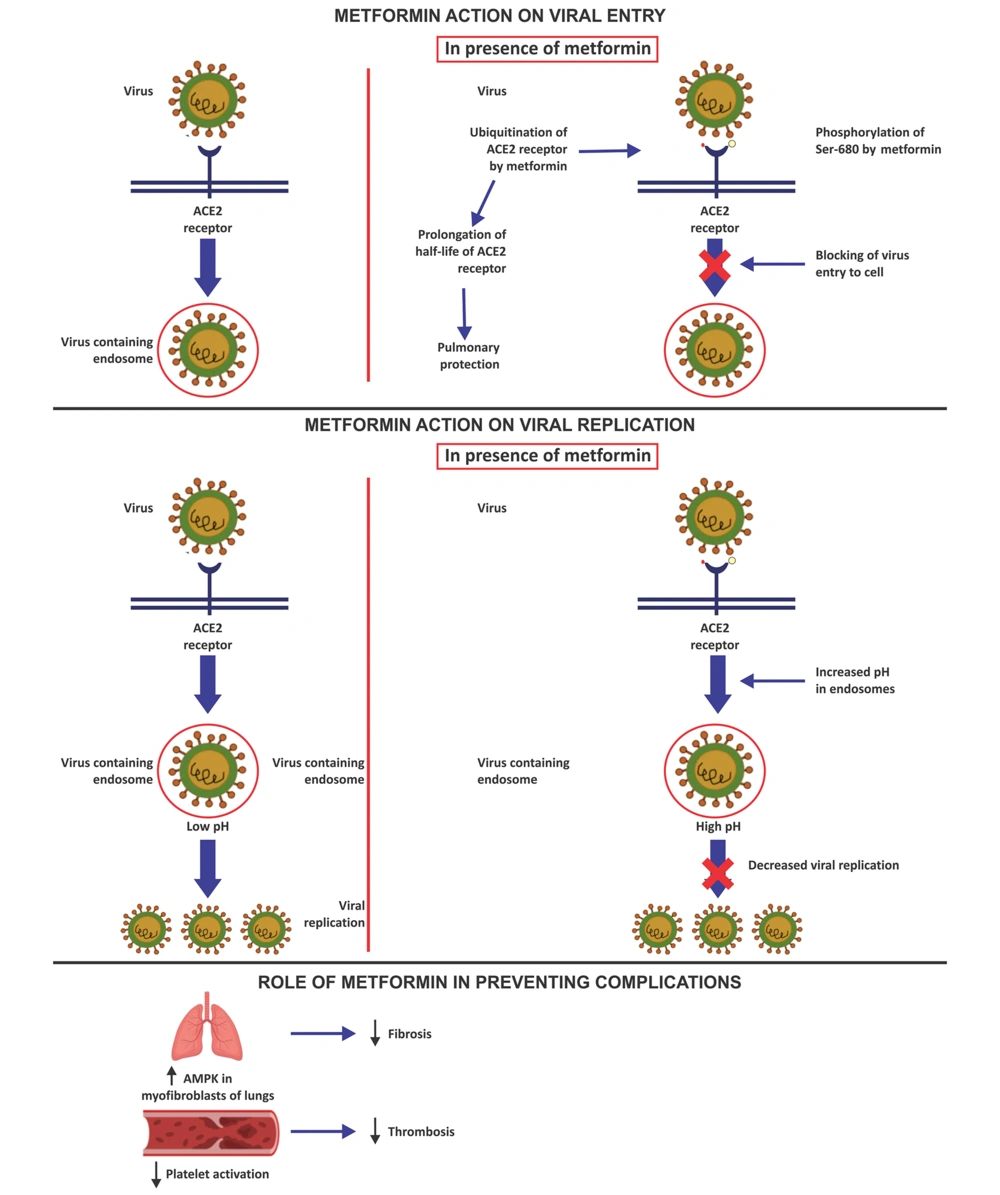
4.3.1. Metformin Action on Viral Entry
Metformin has been proposed to prevent viral entry into respiratory epithelial cells by activating AMP-activated protein kinase (AMPK). Activated AMPK phosphorylates angiotensin-converting enzyme 2 (ACE2) on Ser-680 (23). The addition of a phosphate group is postulated to affect the ACE2 receptor's structure and functionality. A large phosphate moiety could provide steric hindrance and decrease binding with SARS-CoV-2 (24). The post-translational modification of ACE2 is also thought to decrease its ubiquitination and extend its half-life, thereby protecting the lungs (25). Metformin is also presumed to decline the adherence of SARS-CoV-2 to dipeptidyl peptidase-4 (DPP-4), the other postulated receptors of the virus on T cells, by decreasing the levels of circulating DPP-4 (26, 27).
4.3.2. Metformin Action on Viral Replication
The mammalian target of rapamycin (mTOR) protein is involved in the control of cellular metabolism, cell division, and protein synthesis. There is evidence that SARS-CoV-2 infection leads to higher levels of mTOR expression, which promotes virus replication. Metformin suppresses protein synthesis and cell growth by inhibiting the mTOR pathway (28). SARS-CoV-2 entry into the cell involves binding viral spike protein with the ACE2 receptor, followed by internalization of the ACE2 receptor and the virus into the endosome and reprocessing the ACE2 receptors back to the cell surface membrane (29). Endosomal pH plays a crucial role in virus survival inside the host cell. A decreased intracellular pH aids SARS-CoV-2 attachment to the host cell and endosomal virion maturation and proliferation. Endosomal Na+/H+ exchangers (eNHE) and vacuolar ATPase (V-ATPase) are crucial membrane compartments for controlling endosome pH. Higher pH in endosomes caused by metformin's direct action on eNHE and V-ATPase prevents viral multiplication (30).
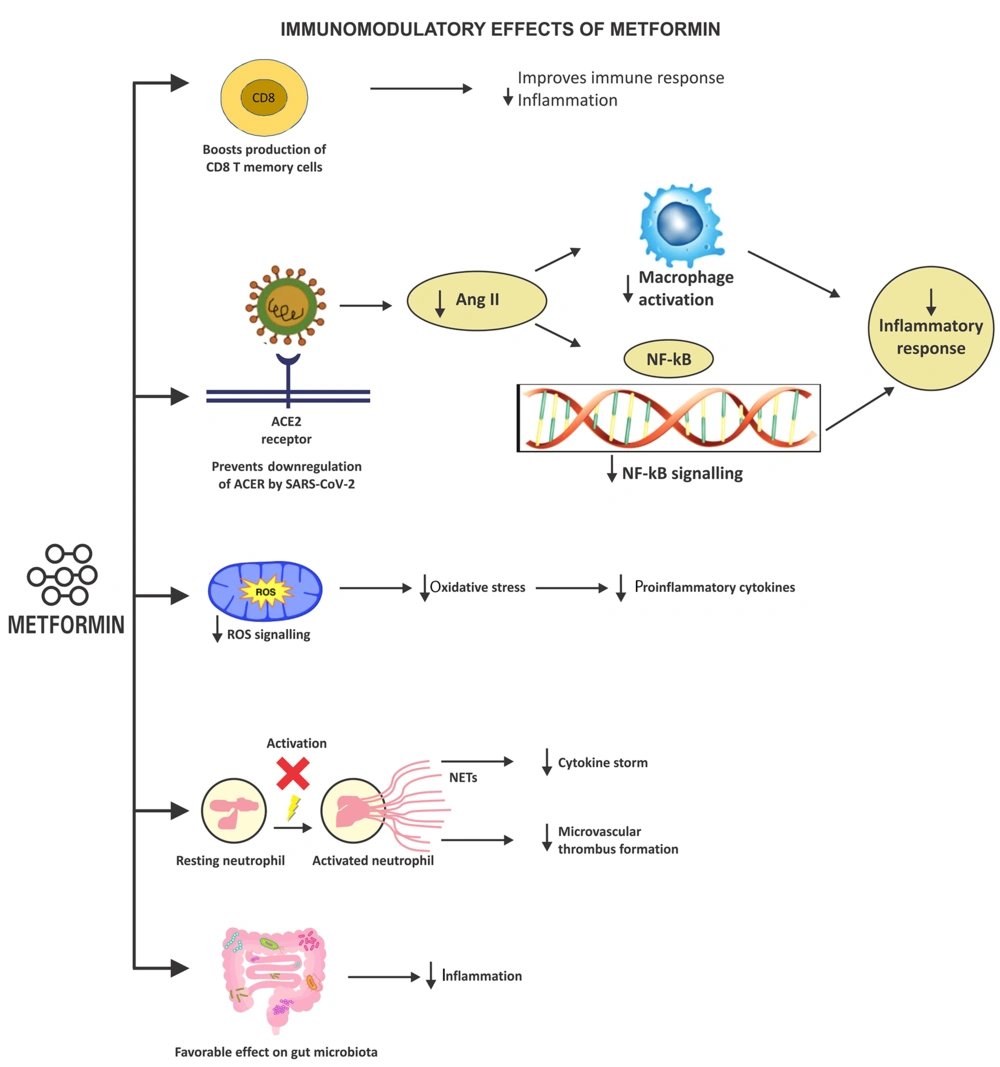
4.3.3. Immunomodulatory Effects of Metformin
Metformin modifies the immune response and decreases inflammation by increasing the generation of CD8+ memory T-cells and T-regulatory cells (31). The ACE2 receptors are downregulated once the virus has been internalized inside the host cell. Increased angiotensin II production causes an imbalance in the renin-angiotensin-aldosterone system (RAAS). Excessive angiotensin II exerts proinflammatory and profibrotic effects and can potentially trigger cardio-pulmonary complications. An activated RAAS pathway in COVID-19 generates inflammatory cytokines, such as NF-κB, that induce severe disease and increases mortality. Metformin prevents these detrimental sequelae by stimulating ACE2 through AMPK-signaling, averting the RAAS imbalance (32). Metformin-mediated overexpression of ACE2 prevents the NF-κB pathway from being activated and stops the production of inflammatory cytokines (12).
The cytokine storm, characterized by an inappropriately exaggerated immune response, generates reactive oxygen species (ROS). Excess ROS is implicated in clinical deterioration and increased fatality in COVID-19 (33). Metformin targets complex I of the mitochondrial electron transport chain (ETC), decreases ROS production, and blocks the release of proinflammatory cytokines mediated by oxidative stress, all of which reduce inflammation-induced immunological responses (34).
The extracellular webs of chromatin, microbicidal proteins, and oxidizing enzymes known as neutrophil extracellular traps (NETs) are secreted by neutrophils to control infections. However, NETs can spread inflammation and microvascular thrombosis in various organs, including the lungs, of patients with acute respiratory distress syndrome (ARDS) when they are not regulated (35). Excessive NET formation leads to a cytokine storm, microthrombi generation, and ARDS in COVID-19. Metformin leads to a decrease in NETs released by neutrophils (36). Metformin also has a favorable effect on the gut flora, which could reduce inflammation (37).
4.3.4. Role of Metformin in Preventing Complications
Deficient AMPK activation is implicated in non-resolving, pathologic fibrotic processes. Metformin accelerated the resolution of well-established fibrosis in an AMPK-dependent way in a mouse bleomycin model of lung fibrosis (38). Decreased platelet activation and risk of thrombosis are also reported with metformin use (12).
4.4. Word of Caution
Despite several theoretical benefits of metformin in combating SARS-CoV-2 infection by the mechanisms mentioned above, metformin does carry some risks. It induces acidosis, especially in patients with impaired renal function (eGFR < 60 mL/min/1.73 m2) or in those on higher doses of metformin (2 - 3 g/day; the risks of acidosis and lactic acidosis rise 12.8 and 22.6 fold, respectively). The acute decline in renal function that affects a significant proportion of patients with COVID-19 and diabetes mellitus is, therefore, a particular concern for the continued use of metformin (39).
5. Limitations
This is a narrative review undertaken to summarize the pathophysiological pathways of the benefits of metformin in COVID-19. Currently, most data on the beneficial effects of metformin are observational and retrospective and should be interpreted with caution. A potential confounding factor that could prejudice results in observational studies is candidates for metformin are in a less advanced stage of diabetes with fewer comorbidities like chronic kidney disease and congestive heart failure. A pragmatic approach would be to continue metformin in patients with diabetes who present with milder forms of COVID-19 but to withdraw when the severity worsens.
6. Conclusions
Combating the harmful effects of SARS-CoV-2 with the pleiotropic actions of metformin seems to be a promising strategy for treating patients with diabetes. In vitro studies have shown complex beneficial interactions of metformin on the various pathophysiological mechanisms in SARS-CoV-2 infection. This can translate into cost-effective and meaningful therapy for people with diabetes who have COVID-19 and should be pursued in further long-term RCTs.