Dear Editor,
Diabetic ketoacidosis (DKA) is the most common hyperglycemic crisis in decompensated diabetes. However, several studies have reported coronavirus disease 2019 (COVID-19) can lead to DKA in non-diabetic patients (1). According to a study, 42 of 659 patients admitted to a hospital with COVID-19 developed DKA, with 27 of these 42 not having a history of diabetes (2). Based on a meta-analysis involving 124,597 children with type 1 diabetes, DKA rates have increased in newly diagnosed diabetic children, and COVID-19 infection can cause DKA in diabetics and non-diabetics, and the length of hospital stay was also positively associated with the development of ketoacidosis (3). Moreover, a study reported that decreased physical activity and irregular follow-up for blood glucose testing during the quarantine aggravated the metabolic condition of both diabetic and non-diabetic individuals. Thus, diabetic patients are at an increased risk of severe COVID-19 (3, 4).
Diabetic ketoacidosis is developed due to the decreased insulin-to-glucagon ratio, leading to decreased glucose uptake, uncontrolled lipolysis, excess ketone body production, metabolic acidosis, and other consequences (5). This condition is inherently pro-inflammatory; however, it is often caused by an underlying disease. The exact underlying mechanism of DKA development in COVID-19 is not clear. The disease is hypothesized to aggravate metabolic dysfunction in asymptomatic diabetic patients or change the body's metabolic defense mechanisms (6).
Role of Hyperglycemia and Pathological Obesity: Hyperglycemia has been frequently observed in critically ill patients due to increased levels of pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α), Interleukin-6 (IL-6), IL-1β, and IL-8. It is worth noting that TNF-α and IL-6 secretions are reciprocally associated with insulin resistance and pathological obesity (7). Moreover, adipokines, a group of hormones secreted by adipose tissue, contribute to insulin resistance by showing pro- or anti-inflammatory properties (8). Adipose tissue secretes TNF- and IL-6, inhibiting glucose uptake and impairing insulin signaling. As a result, the inflammation induced by metabolic dysfunction is highly important in developing insulin resistance in obese individuals, increasing the COVID-19-induced cytokine release in these patients (9). Also, the excess visceral adiposity in obese patients can increase the mitochondrial reactive oxygen species (ROS) production, IL-6, and C-reactive protein (CRP) levels. Therefore, the oxidative stress induced by elevated levels of ROS during the infection may exacerbate this inflammatory condition. Finally, the infection can activate the toll-like receptor 4 (TLR4) pathway, aggravating the host's inflammatory response (via the MyD88 dependent or independent pathway) (10).
Under hyperglycemic conditions, excess ROS production causes increased glycolysis and protein kinase C induction, exacerbating cell injury and apoptosis that enhance cellular adhesion and lead to coagulopathy. Generally, the pre-existed metabolic imbalance can be aggravated by infection due to increased oxidative stress (11).
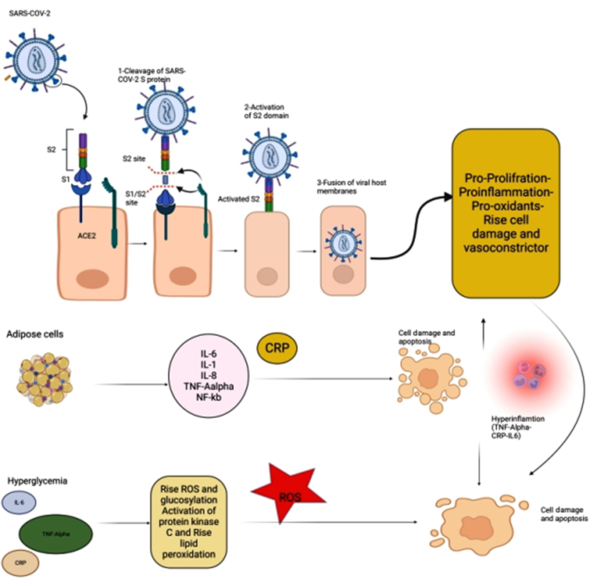
Role of ACE2 Ligand Induction: Typically, the SARS-CoV-2 envelope contains several proteins, including the membrane (M), envelope (E), and spike (S) proteins (12). Among these, the S protein, consisting of two subunits of S1 and S2, is used for viral entry into host cells. During this process, the S1 subunit binds to one of its receptors on the cellular membrane, such as angiotensin-converting enzyme-2 (ACE2), CD26, ezrin, and cyclophilins. Afterward, the S2 subunit allows the entry of the viral genome into the host cell by fusing the viral envelope with the host cell membrane. Therefore, ACE2 has a crucial influence on the pathology of COVID-19. Consequently, the cells expressing high levels of ACE2 on their membranes are more vulnerable to infection and subsequent cell damage (13).
The receptor binding domain (RBD) has a crystal structure for binding the virus to the ACE 2 receptor. Most antibodies block this region, but with the evolution of the virus, the effect of this antibody decreases. According to the latest research, the external region RBD that does not bind to the ACE2 receptor can be a potential target for new antibodies (SW186) (14).
Angiotensin-converting enzyme-2 is widely expressed in various tissues, including the lungs, intestines, liver, pancreas, and macrophages. Moreover, it has been shown that ACE2 expression by SARS-CoV-2 may have several consequences, including hypertension, diabetes, and cardiovascular diseases. Such a situation can indirectly facilitate apoptosis by creating α-TNF, increasing serotonin expression, and stimulating leukocyte immunoglobulin-like receptor subfamily B member 3 (LiLRB3), interfering with the activity of β cells in the pancreas. Also, it is hypothesized that overactivation of the renin-angiotensin-aldosterone system (RAAS) can lead to impaired glucose uptake and insulin resistance, resulting in hyperglycemia and increased oxidative stress due to elevated ROS production (15).
The binding of SARS-CoV-2 to ACE2 causes an overproduction of angiotensin II, stimulating the NF-κB and IL-6 pathways. Subsequently, the NF-κB pathway can aggravate the inflammatory state. Thus, viral binding to ACE2 can lead to hyperglycemia and excessive oxidative stress caused by increased ROS production, disrupting the most important factors involved in RAAS homeostasis and leading to DKA in patients with COVID-19 (Figure 1) (16).
Considering the above, evaluating the metabolic and inflammatory abnormalities in COVID-19 patients may provide the researchers with the necessary information for developing effective therapeutic strategies for decreasing the morbidity and mortality of DKA in these patients. These therapeutic options include targeted therapies or anti-inflammatory, antiviral, immunomodulatory, or antioxidant medications. In general, there is a need for further studies in this field investigating the possible underlying mechanisms that may confirm or disprove these hypotheses.