1. Background
The World Health Organization in March 2020 announced coronavirus disease 2019 (COVID-19) as a pandemic and severe respiratory illness (1). COVID-19 can include a wide range of symptoms, from only flu-like symptoms to severe infection with advanced complications such as cardiovascular and diabetes complications (2, 3). On the other hand, several factors, such as older age, male sex, and diabetes, were identified as worse outcomes for COVID-19 (4). Diabetes mellitus is one of the most common comorbidities with COVID-19 (5). Based on a whole-population study in England, diabetes is responsible for a third of mortality due to COVID-19 (6). One possible reason for increased in-hospital deaths with COVID-19 in individuals with diabetes is the complication of diabetic ketoacidosis (DKA) (7).
Diabetic ketoacidosis is a hyperglycaemic emergency that typically occurs in patients with type 1 diabetes mellitus (T1DM) because of different causes, such as insulin deficiency and infection (8). There is evidence that DKA may increase during the pandemic. This event can occur due to avoiding accessing the hospital due to fear of getting infected, poor provision of care by health care providers, or the pathogenesis mechanism of the virus (9, 10).
Although evidence suggests a relationship between COVID-19 and DKA, there are conflicts, and more research is needed. Several studies reported increased number of new-onset DKA among children with T1DM (11, 12). However, some studies did not observe any substantial relationship between COVID-19 and an increase in the rates of DKA (13). Additionally, most studies are related to T1DM, and a few studies have evaluated the condition of DKA in type 2 diabetes mellitus (T2DM). On the other hand, the study on DKA during COVID-19 in Iran is limited, and there are only a few case-report studies (14-16).
2. Objectives
This study aimed to assess new-onset DKA status in patients with T1DM and T2DM before and during COVID-19.
3. Methods
3.1. Study Design and Participants
This study was approved by the Institutional Ethics Committee of Shahid Sadoughi University of Medical Sciences, Yazd, Iran (the code: IR.SSU.REC.1400.142). In this single-center retrospective-longitudinal study, we used data from the Shahid Sadoughi Hospital in Yazd, Iran, to identify individuals hospitalized due to DKA between Mar 1 and Aug 31, 2018, 2019, 2020, and 2021. The periods of 2018 and 2019 were considered the time before the conflict with COVID-19, and the periods of 2020 and 2021 were considered the time during the conflict with COVID-19 in Iran. All patients’ information was extracted from their paper files and electronic medical and then included in the preparation checklist.
3.2. Procedures
All patients entered the study phase according to hospital admissions with the code related to DKA and diabetes type (E10.1 and E11.1). The E10.1 code is defined for diagnosis of T1DM with ketoacidosis and the E11.1 code is for diagnosis of T2DM with ketoacidosis.
DKA was defined as blood glucose higher than/ or equal to 250 mg/dL, pH level less than 7.3, and/or bicarbonate level less than 15 mmol/L; also, the first appearance of clinical symptoms for DKA was defined as new-onset DKA (11).
Demographic information of patients included age, gender, national code, history of diabetes, history of COVID-19 (based on a real-time polymerase chain reaction (RT-PCR)), history of DKA, and severity of DKA. The severity of DKA was defined when the following three criteria were met (17):
(1) Mild: Plasma glucose (mg/dL) > 250, arterial pH 7.25 - 7.30, serum bicarbonate (mEq/L) 15 - 18, and mental status of alert;
(2) Moderate: Plasma glucose (mg/dL) > 250, arterial pH 7.00 - < 7.24, serum bicarbonate (mEq/L) 10 - < 15, and mental status of alert/drowsy;
(3) Severe: Plasma glucose (mg/dL) > 250, arterial pH < 7.00, serum bicarbonate (mEq/L) < 10 and mental status of stupor/coma.
3.3. Statistical Analysis
Demographic and clinical characteristics were reported as means ± standard deviation (SD) or as percentages. The chi-square test was used to calculate the statistical significance of categorical variables. The Student’s t-test was used to compare the means between two groups, and ANOVA was used to compare the means among four groups (periods before and after COVID-19). Moreover, Mann‐Whitney and Kruskal Wallis tests were used for nonparametric variables. All analyses were performed using SPSS 22. A P-value less than 0.05 was considered statistically significant.
4. Results
In 162 patients with DKA, 139 (85.5%) were newly diagnosed. Among participants, 77 were female (55.4%), and 62 were male (44.6%), with an average age of 22.87 ± 20.48 years. Additionally, the mean of days of hospitalization for individuals with new-onset DKA obtained 4.53 ± 3.50.
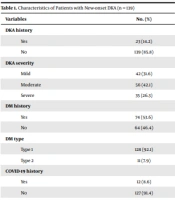
Table 1 shows the characteristics of studied patients with new-onset DKA. As shown in Table 1, 53.6% of patients were pre-existing diabetes, and 8.6% of new-onset DKA had a positive RT-PCR test for COVID-19. According to hospital admissions with the code related to DKA type, 7.9% of the patients belonged to patients with T2DM. Moreover, laboratory data related to participants have been brought in Table 2.
| Variables | No. (%) |
|---|---|
| DKA history | |
| Yes | 23 (14.2) |
| No | 139 (85.8) |
| DKA severity | |
| Mild | 42 (31.6) |
| Moderate | 56 (42.1) |
| Severe | 35 (26.3) |
| DM history | |
| Yes | 74 (53.6) |
| No | 64 (46.4) |
| DM type | |
| Type 1 | 128 (92.1) |
| Type 2 | 11 (7.9) |
| COVID-19 history | |
| Yes | 12 (8.6) |
| No | 127 (91.4) |
| Outcome | |
| Alive | 133 (96.4) |
| Death | 5 (3.6) |
Characteristics of Patients with New-onset DKA (n = 139)
| Laboratory Tests | Mean ± SD |
|---|---|
| PH | 7.16 ± 0.155 |
| HCO3 (mmol/L) | 8.54 ± 5.71 |
| Plasma glucose (mg/dL) | 444.28 ± 144.99 |
| pCO2 (mmHg) | 20.66 ± 8.65 |
| Urea (mg/dL) | 42.18 ± 41.24 |
| Cr (mg/dL) | 1.23 ± 0.83 |
Laboratory Data Related Patients with New-onset Diabetic Ketoacidosis (n = 139)
Our results showed that the frequency of individuals who were new-onset DKA increased significantly during the COVID-19 pandemic compared to pre-COVID-19 (P = 0.002). Interestingly, it was found that the frequency of new-onset of diabetes in patients who were hospitalized due to DKA increased significantly during the COVID-19 pandemic compared to before COVID-19 (P = 0.047) (Table 3).
| Variables | 2018 Before COVID | 2019 Before COVID | 2020 During COVID | 2021 During COVID | P-Value |
|---|---|---|---|---|---|
| Age | 23.17 ± 19.14 | 31.48 ± 23.15 | 21.66 ± 21.28 | 19.98 ± 18.79 | 0.151 b |
| Sex | 0.954 c | ||||
| Female | 14 (60.9) | 12 (52.2) | 21 (55.3) | 30 (54.5) | |
| Male | 9 (39.1) | 11 (47.8) | 17 (44.7) | 25 (45.5) | |
| New-onset DKA | 23 (71.9) | 23 (74.2) | 38 (92.7) | 55 (94.8) | 0.002 c |
| Days of hospitalization | 4 ± 2.11 | 5.78 ± 4.51 | 2.26 ± 3.26 | 4.40 ± 3.62 | 0.291 b |
| DKA severity | |||||
| Mild | 10 (45.5) | 10 (47.6) | 13 (37.1) | 9 (16.4) | 0.000 c |
| Moderate | 9 (40.9) | 10 (47.6) | 17 (48.6) | 20 (36.4) | |
| Severe | 3 (13.6) | 1 (4.8) | 5 (14.3) | 26 (47.3) | |
| DM history | 0.047 c | ||||
| Yes | 15 (65.2) | 17 (73.9) | 19 (50) | 23 (42.6) | |
| No | 8 (34.8) | 6 (26.1) | 19 (50) | 31 (57.4) | |
| DM type | < 0.001 c | ||||
| Type 1 | 23 (100) | 23 (100) | 38 (100) | 44 (80) | |
| Type 2 | 0 | 0 | 0 | 11 (20) | |
| Outcome | 0.981 c | ||||
| Alive | 22 (95.7) | 22 (65.7) | 37 (97.4) | 52 (96.3) | |
| Death | 1 (4.3) | 1 (4.3) | 1 (2.6) | 2 (3.7) | |
| Laboratory tests | |||||
| pH | 7.20 ± 0.15 | 7.23 ± 0.11 | 7.18 ± 0.15 | 7.10 ± 0.15 | 0.001 d |
| HCO3 (mmol/L) | 9.17 ± 4.15 | 12.07 ± 5.95 | 8.45 ± 5.86 | 6.72 ± 5.40 | 0.000 b |
| Plasma glucose (mg/dL) | 383.74 ± 145.66 | 450.30 ± 151.45 | 431.43 ± 142.24 | 476.91 ± 138.03 | 0.069 d |
| pCO2 (mmHg) | 22.31 ± 6.69 | 26.43 ± 7.68 | 19.44 ± 7.85 | 18.46 ± 19.19 | 0.001 d |
Characteristics of Patients with New-onset Diabetic Ketoacidosis
Moreover, the severity of new-onset DKA significantly increased during the COVID-19 pandemic compared to patients with newly diagnosed DKA during pre-pandemic (P = 0.000). On the other hand, of a total of 139 patients with new-onset DKA, 13.6% and 4.8% of individuals have been detected with severe DKA during the studied periods of 2018 and 2019 respectively; meanwhile, this frequency obtained 14.3% and 47.3% for the same period in 2020 and 2021 respectively. However, there was no significant difference between pre-pandemic and pandemic in terms of the final outcome (death vs. discharge) (P = 0.981).
Interestingly, according to hospital admissions with the code related to DKA type, no T2DM patients with DKA were reported in the considered periods before the pandemic, but DKA admissions in people with T2DM increased by 20% in 2021 (P < 0.001).
Next, we compared patients with new-onset DKA during COVID-19 based on diabetes type (Table 4). Analysis of the results showed there was a significant difference in terms of hospitalization days (P = 0.043) and disease outcome (death vs. discharge) (P = 0.038) between patients with T1DM and T2DM in 2021. Although there was no significant difference between type 1 and type 2 patients considering the severity of DKA (P = 0.121), hospitalization period and mortality in type 2 patients were higher than in type 1. On the other hand, the results of laboratory tests showed there was a significant difference in terms of HCO3 (P = 0.003) and pCO2 (P = 0.002). But there was no significant difference between the two groups considering plasma glucose (P = 0.708) and pH (P = 0.402).
| Variables | Type 1 (n = 44) | Type 2 (n = 11) | P-Value |
|---|---|---|---|
| Age (y) | 12.26 ± 10.67 | 50.63 ± 11.17 | < 0.001 b |
| Sex | 0.028 c | ||
| Female | 21 (47.7) | 9 (81.8) | |
| Male | 23 (52.2) | 2 (18.2) | |
| Days of hospitalization | 3.75 ± 2.81 | 7 ± 5.25 | 0. 043 b |
| DKA severity | 0.121 c | ||
| Mild | 5 (11.4) | 4 (36.4) | |
| Moderate | 18 (40.9) | 2 (18.1) | |
| Severe | 21 (47.7) | 5 (45.5) | |
| DM history | 0.039 c | ||
| Yes | 15 (34.9) | 8 (72.7) | |
| No | 28 (65.1) | 3 (27.3) | |
| COVID-19 history (RT-PCR test) | 0.134 c | ||
| Yes | 4 (9.1) | 3 (27.3) | |
| No | 40 (90.9) | 8 (72.7) | |
| Outcome | 0.038 c | ||
| Alive | 43 (100) | 9 (81.8) | |
| Death | 0 (0) | 2 (18.2) | |
| Laboratory tests | |||
| pH | 7.09 ± 0.15 | 7.13 ± 0.16 | 0.402 d |
| HCO3 (mmol/L) | 6.12 ± 5.60 | 9.15 ± 3.78 | 0.003 b |
| Plasma glucose (mg/dL) | 480.60 ± 139.50 | 462.82 ± 137.90 | 0.708 d |
| pCO2 (mmHg) | 16.61 ± 8.43 | 25.86 ± 8.69 | 0.002 d |
Characteristics of Patients with New-onset Diabetic Ketoacidosis During Coronavirus Disease 2019 Based on Diabetes Type
Additionally, to find out what percentage of the studied patients had a history of COVID-19 before a diagnosis of DKA, we analyzed data based on a history of COVID-19. Evaluation of COVID-19 status in patients with new-onset DKA based on the RT-PCR test showed that 9.1% of patients with T1DM and 27.3% with T2DM were RT-PCR positive, but this difference was insignificant (P = 0.134) and in 2020, four patients (10.52%) were positive for the RT-PCR test.
5. Discussion
The results of this study showed that the frequency and severity of DKA among individuals with new-onset DKA was higher during the pandemic than pre-pandemic. Significantly, no patients with T2DM and new-onset DKA were observed in the pre-pandemic, but DKA admissions in patients with T2DM increased during the pandemic.
Today, viral infections such as seasonal influenza infect millions of people every year and lead to the death of thousands of people. However, these viruses still remain a problem, and viral pandemics such as COVID-19 are likely to recur every decade (18). Therefore, identifying and managing COVID-19 complications is essential.
Epidemiological studies have shown that people with diabetes are at risk of worsening COVID-19 clinical outcomes. On the other hand, evidence suggests COVID-19 may lead to diabetes (10).
Our study showed that new-onset diabetes mellitus (DM) has significantly increased during the COVID-19 pandemic. A study by Chambers et al. demonstrated that the number of pediatric new-onset DM has increased in the United States during the pandemic (19). Another study showed that the incidence rate ratio of new-onset T1DM during the COVID-19 pandemic has increased in children in Finland (4). It has been found that the coronavirus enters host cells mainly through ACE2 receptors. Besides, beta cells in the pancreas contain large amounts of ACE2 receptors. As a result, there is a theory that COVID-19 may also affect beta cells in the pancreas by ACE2 receptors and lead to new-onset DM (20). It is suggested that when the new-onset DM increases during a period, the risk of DKA increases (21).
DKA occurs in the setting of insulin deficiency or a state of infectious disease which infection is the most common reason and leads to lipolysis and is followed by the increased production of ketone bodies (6). This study revealed that during the COVID-19 pandemic, the frequency of new-onset DKA was significantly more than before COVID-19. A retrospective cohort study suggested that COVID-19 can accelerate lipolysis and cause ketosis or ketoacidosis (7). COVID-19 can lead to DKA directly through its pathogenesis mechanisms or indirectly. It has been revealed that interleukin-6 levels increase in patients with COVID-19. Interleukin-6 acts as an inducer of ketogenesis and increases DKA. This evidence suggests that the frequency of newly diagnosed DKA may increase during COVID-19 and coronavirus-provoked ketosis-prone diabetes. On the other hand, in addition to the pathological mechanism of the coronavirus, the evidence shows that the emergence of a pandemic can affect mental health and leads to stress among people, hospital avoidance, and disruption of medical care (22, 23). DKA can occur as a consequence of delayed diagnosis or treatment. One of the protective effects of DKA is having a first-degree relative with DM due to increased knowledge and access to medical care (24). Consequently, awareness of healthcare workers and families about COVID-19 complications such as DKA results in better management of diseases (25).
Dzygalo et al., intending to evaluate the severity of DKA during COVID-19, found that the rate of severe DKA in children with T1DM has increased during the COVID-19 pandemic (21). Another study in Australia showed that the frequency of severe DKA among patients with newly diagnosed T1DM was remarkably higher during the pandemic compared to before the pandemic (26). The results of this study were in line with the mentioned studies and showed the severity of DKA was significantly higher during the pandemic than before. A study in Italy found that due to fear of COVID-19, people refer to the hospital less often, which can lead to severe DKA due to delayed access to hospital care (9). On the other hand, a multi-center study showed that a delay of more than 24 hours in treating patients with DKA leads to the progression of the disease (27). So, it seems that early diagnosis and timely treatment can reduce the severity of the disease.
Although most of the studies conducted on DKA during the COVID-19 pandemic are related to patients with T1DM (21, 28) and few studies investigated DKA in T2DM (29, 30), our findings showed an unusual proportion in the frequency of DKA in adults with T2DM during the pandemic, which indicates individuals with T2DM are prone to DKA. In the study at a German University Hospital, it was reported that two adolescent patients with newly diagnosed T2DM were admitted because of their DKA in 2020 (during the pandemic); however, in 2019, before the pandemic, no adolescent with T2DM was reported (30). Another study at Children’s Hospital Los Angeles reported that in pediatric T2DM, the incidence of DKA has increased during the pandemic (29). The average mean age in patients with T2DM in this study was 50.63 ± 11.17, which shows patients with newly diagnosed DKA were mature, and none were adolescent or pediatric. These findings emphasize the importance of further investigations in different populations with age group classification. During selected study periods, no individual with T2DM with DKA complications was seen except in 2021. To delve deeper into the investigation, we assessed the frequency of DKA in people with T2DM who had been admitted from March 2017 to February 2020 at Shahid Sadoughi Hospital. It is revealed that two patients with T2DM in the months of January and February of 2020 were admitted to the hospital because of DKA, and interestingly none of the patients with T2DM had been admitted for DKA in 2017 - 2019. Comparison of type 1 and type 2 diabetes with new-onset DKA in 2021 showed that even though there was no significant difference in terms of the severity of DKA, the hospitalization period, as well as the frequency of death, were remarkably more among patients with T2DM compared to T1DM. These findings indicate that patients with T2DM have a more complex need for hospital care. Given that the occurrence of DKA in T2DM is commonly related to conditions of extreme stress, paying attention to the complications of DKA in patients with T2DM during the pandemic period is crucial (31). It is unknown whether the rise in DKA, especially in T2DM, is related to COVID-19 exposure or poor management of blood sugar control due to staying at home. According to the previous study, patients with diabetes avoided going to crowded places like hospitals during the pandemic (32). As a result, proper control of blood sugar can largely prevent the occurrence of the disease and its complications.
Assessment of the history of COVID-19 showed that 8.6% of studied patients had a history of COVID-19 based on an RT-PCR test. In contrast to other respiratory viruses, severe COVID-19 is less common in children and young adults rather than in older adults (33). Consequently, since most of the patients in this study were young, it is possible that they did not have a severe form of the disease and did not have any tests. Therefore, it is better to investigate the causes of the pathogenesis of this disease in future studies.
Moreover, we compared laboratory outcomes for patients with new-onset DKA pre- and during COVID-19. Our results showed that DKA-related tests, including pH, HCO3, and pCO2, were significantly different during COVID-19 than before.
Due to differences in laboratory observations and disease outcomes, it is suggested that DKA may be distinct from before the pandemic and considered a novel presentation (34). As a consequence, healthcare workers should pay special attention to DKA during the pandemic. Also, the results from this study highlight that when determining COVID-19 treatment strategies, attention should be paid to DKA, especially in people with T2DM.
This study suffers from several limitations. Firstly, considering that the study was conducted only in the reference hospital, this may be known as a bias that may influence the observed differences in our results. Secondly, socioeconomic disadvantage, high HbA1c, young age, and female sex are known to increase the risk of ketoacidosis in diabetes (35); in this study, we did not have access to the HbA1c and their socioeconomic status, so it is suggested that in future studies these factors be assessed. Moreover, the design of this study was based on international codes, which can miss euglycemic DKA, so it is suggested that in future studies, these individuals are considered. Thirdly, the test results for COVID-19 were only based on RT-PCR data, and the results of the CT scan and serology of the patients were unavailable.
In conclusion, our results showed that during the COVID-19 pandemic, the frequency of DKA and its severity was higher than before the pandemic, and the COVID-19 pandemic can be more life-threatening in patients with T2DM. Therefore, physicians and healthcare providers should be alert to DKA, especially in patients with T2DM.