1. Background
Infertility is a common health concern around the world. Infertility is the failure to conceive after at least a year of regular unprotected sexual intercourse (1, 2). It is estimated that more than 186 million people worldwide are infertile (3). According to a recent meta-analysis of population-based studies, the overall prevalence of infertility was 7.88% among the Iranian population (4). In a study, the prevalence of infertility was reported at 24.58% among the Chinese population (5). Male factors are the primary cause of infertility in approximately one-third of couples (1, 2). According to the study by Agarwal et al., the prevalence of male infertility in Sub-Saharan Africa was (2.5% to 4.8%), followed by Central/Eastern Europe (8% to 12%), North America (4.5% to 6%), and Australia (8% to 9%) (6).
Various factors can influence male fertility, of which some factors have been reported as possible causes and risk factors, including biological, physiological, genetic, behavioral/lifestyle, environmental, and sociodemographic risk factors (7). Furthermore, it has been shown that there is a strong link between metabolic abnormality and infertility (8, 9). Infertility and metabolic syndrome (MetS) share a common risk factor (10). The production of reactive oxygen species (ROS) and increased oxidative stress (OS), endothelial dysfunction, and altered semen quality might be occurred due to metabolic abnormality (10).
Although several studies have reported the prevalence of male infertility, most have been conducted on the population who referred to infertility centers (11-13). Furthermore, there is limited evidence among the Iranian population that highly focuses on the metabolic determinants of male infertility. Hence, our study aimed to investigate the prevalence and metabolic determinants of self-reported male infertility among participants in the Tehran Lipid and Glucose Study (TLGS).
2. Objectives
This study aimed to examine the prevalence of self-reported male infertility and related metabolic disturbance.
3. Methods
3.1. Ethics Approval
This study was approved by the Medical Ethics Committee of the Endocrine Sciences Research Institute, Shahid Beheshti University of Medical Sciences (code: IR.SBMU.ENDOCRINE.REC.1398.007). Written informed consent was obtained from all participants.
3.2. Subjects
Participants were selected from the TLGS. TLGS is a cohort study conducted to evaluate the prevalence and associated factors for non-communicable conditions. There are previously published details of TLGS (14-16). The current study employed data from the sixth visit of the TLGS (2015 - 2018), comprising detailed information on individuals' fertility status (16).
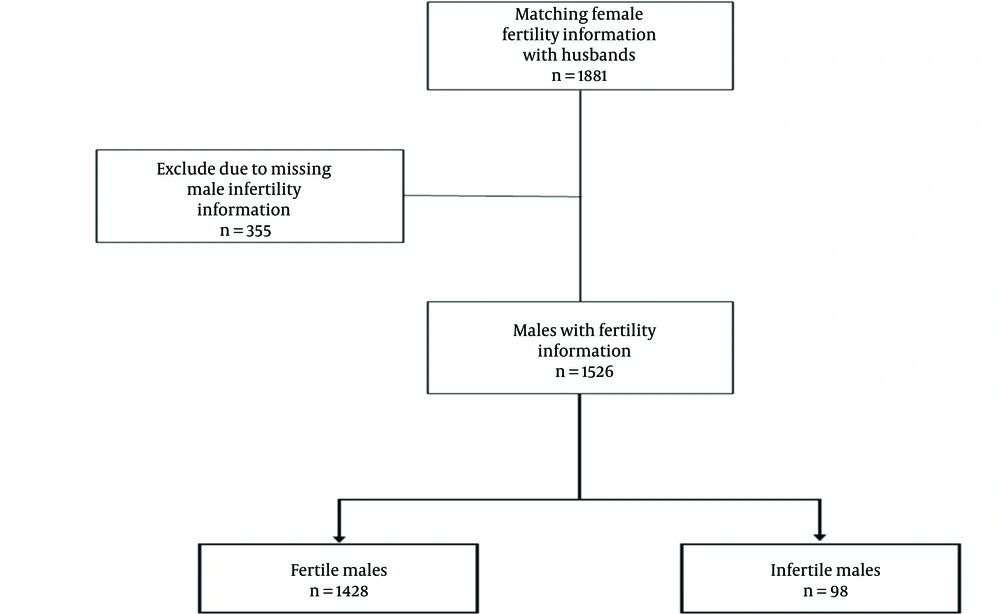
Inclusion criteria included individuals who had been married or had not documented female infertility. The fertility information of 1881 fertile females was matched with their husbands. After excluding participants with missing male infertility information, 1526 males were included in this study (Figure 1).
3.3. Measurements
A professional team measured clinical, anthropometric, and biochemical parameters. The body weight was measured on a digital scale (Seca 707, Seca GmbH) while wearing the least amount of clothes, and the result was rounded to the closest to 100 g. Similarly, the shoulders' natural posture and height without shoes in the standing position were measured using a meter. The following formula was used to determine the body mass index (BMI): Weight in kilograms divided by height in meters squared (kg/m2).
A tape measure was used to measure the waist circumference (WC) without applying pressure to the body's surface or tension to the umbilical surface. Without applying any pressure on the body's surface, the anterior-superior iliac spine was used to measure the hip circumference (HC). We also measured the blood pressure twice (after 15 minutes of rest) on the arm while seated using a conventional mercury sphygmomanometer. The average of these readings was then noted.
Between 7 AM and 9 AM, the blood samples were collected following a 12-hour fast. All samples were kept at -80°C until analysis. By employing glucose oxidase as an enzyme, we assessed fasting plasma glucose (FPG). Total cholesterol (TC) was determined by an enzymatic colorimetric method using cholesterol esterase and cholesterol oxidase. Glycerol phosphate was used to determine the levels of serum triglyceride (TG). High-density lipoprotein cholesterol (HDL-C) levels were measured after the deposition of apolipoprotein B (apoB) containing lipoproteins using phosphotungstic acid. We used a modified Friedewald formula to estimate low-density lipoprotein cholesterol (LDL-C) levels (17). All metabolic parameters were evaluated using relevant kits (Pars Azmoun, Tehran, Iran) and Selectra 2 autoanalyzers (Vital Scientific, Spankeren, the Netherlands). The controls of lyophilized serum in the normal and pathological ranges were used to check the assay's performance after every 25 trials, and all samples were evaluated once the internal quality control passed muster (18, 19).
3.4. Definitions
Infertility occurs when a couple is unable to conceive after engaging in unprotected sex for at least 1 year (20, 21). In the current study, data on male infertility were gathered by reviewing the history of infertility using a self-report questionnaire and then verified by further medical records.
Metabolic syndrome was defined as having at least 3 of the following 5 criteria: TG level ≥ 150 mg/dL, taking a certain medication, or HDL ≤ 40 mg/dL; diastolic blood pressure (DBP) 85 mmHg, systolic blood pressure (SBP) ≥ 30 mmHg, or the use of a specific medication; FPG ≥ 100 mg/dL or receive special treatment (22); WC ≥ 90 cm for males according to the specific population threshold of Iran. Dyslipidemia was defined as hypertriglyceridemia (TG ≥ 150 mg/dL), hypo-HDL (HDL < 40 mg/dL), and/or using lipid-lowering drugs (23). Also, obesity was defined as BMI ≥ 30 kg/m2, and central obesity was defined as WC ≥ 90 cm (24).
The criteria for underweight, normal weight, overweight, and obese were based on BMI (kg/m2) and the following WHO and the National Heart, Lung, and Blood Institute (NHLBI) definitions: Underweight < 18.5, normal weight 18.5 to 24.9, overweight 25 to 29.9, obese 30 or more kg/m2 (25, 26).
Type 2 diabetes mellitus was defined as FPG ≥ 126 mg/dL, 2-hour post-challenge plasma glucose (2h-PCPG) values in the oral glucose tolerance test (OGTT) that were 200 mg/dL, or utilizing an anti-diabetic medicine (27). A set of heart and blood vessel problems is referred to as cardiovascular disease (CVD). Coronary heart disease, cerebrovascular disease, peripheral arterial disease, rheumatic heart disease, congenital heart disease, deep vein thrombosis, and pulmonary embolism are examples of these conditions (28).
3.5. Statistical Analysis
Participants' initial traits were reported and contrasted in the groups of fertile and infertile males. We used the Kolmogorov-Smirnov test to determine the normality of the continuous variables. Mean ± SD and the Mann-Whitney U test were employed to characterize and compare continuous data if the normality assumption was rejected. Mean (SD) and the independent Student t test were utilized when the normality assumption was not rejected. The chi-square or Fisher exact test was used to compare the categorical variables, expressed as frequencies (%; for tables with sparse cells).
Statistical analysis was executed using the Logistic regression model. The coefficients obtained from the model's fit for each independent variable are interpreted as the odds ratio (OR) of that variable in the outcome occurrence. Model 1 was an unadjusted model. Model 2 was adjusted for age and BMI. Model 3 (fully adjusted) was adjusted for variables in model 2 plus WC, HC, SBP, DBP, FPG, TC, TG, HDL, LDL, educational level, smoking history, diabetes mellitus, MetS, dyslipidemia, CVD, obesity, normal weight, overweight, and obese. Data analysis was performed using R version 4.1.1 and SPSS version 21 (SPSS Inc., Chicago, IL, USA). P values less than 0.05 were considered statistically significant.
4. Results
A total of 1526 subjects were enrolled in this study. Males with self-reported infertility (n = 98) and fertile males with at least 1 live birth (n = 1428). The total prevalence of self-reported male infertility was 6.42% (98/1526). The median [interquartile range (IQR)] age of the sample was 55 (47 - 63) years. In both the fertile and infertile groups, most individuals were between 40 and 50 years old. The mean (SD) of BMI was 26.80 (3.93) and 26.92 (4.36) kg/m2 in fertile and infertile participants, respectively. The overview of the participants' initial characteristics is shown in Table 1, suggesting that there was no significant difference between the 2 groups in any of the covariates in the study.
| Variables | Fertile (n = 1428) | Infertile (n = 98) | P Value a |
|---|---|---|---|
| Age (y) | 0.217 | ||
| < 40 | 285 (20) | 21 (21.4) | |
| 40 - 50 | 642 (45) | 51 (52) | |
| > 50 | 501 (35) | 26 (26.5) | |
| BMI (kg/m2) | 26.80 ± 3.93 | 26.92 ± 4.36 | 0.793 |
| WC (cm) | 94.86 ± 11.92 | 94.79 ± 10.50 | 0.851 |
| HC (cm) | 97.15 ± 8.13 | 97.89 ± 7.82 | 0.424 |
| SBP (mm Hg) | 120.58 ± 17.52 | 121.62 ± 17.52 | 0.436 |
| DBP (mm Hg) | 78.74 ± 10.66 | 79.50 ± 10.14 | 0.311 |
| FPG (mg/dL) | 106.15 ± 37.30 | 103.31 ± 29.98 | 0.752 |
| TC (mg/dL) | 186.52 ± 40.26 | 193.90 ± 35.59 | 0.077 |
| TG (mg/dL) | 159.86 ± 97.00 | 165.88 ± 74.61 | 0.078 |
| HDL-C (mg/dL) | 42.08 ± 9.71 | 41.94 ± 9.25 | 0.622 |
| LDL-C (mg/dL) | 113.23 ± 34.71 | 119.20 ± 33.56 | 0.099 |
| Educational level (y) | 0.389 | ||
| < 6 | 23 (1.6) | 0 (0) | |
| 6 - 12 | 1048 (73.6) | 75 (77.3) | |
| > 12 | 352 (24.7) | 22 (22.7) | |
| Smoking history (yes) | 647 (45.3) | 44 (44.9) | 0.937 |
| Dyslipidemia (yes) | 1275 (89.3) | 82 (83.7) | 0.087 |
| Diabetes mellitus (yes) | 320 (22.4) | 19 (19.4) | 0.486 |
| MetS (yes) | 883 (61.8) | 60 (61.2) | 0.958 |
| Obesity | 0.752 | ||
| Underweight | 23 (1.6) | 3 (3.1) | |
| Normal weight | 404 (28.3) | 27 (27.6) | |
| Overweight | 679 (47.5) | 47 (48.0) | |
| Obese | 322 (22.5) | 21 (21.4) | |
| CVD (yes) | 247 (17.3) | 10 (10.2) | 0.070 |
Abbreviations: BMI, body mass index; WC, waist circumference; HC, hip circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MetS, metabolic syndrome; CVD, cardiovascular disease.
a The comparison P value between groups was calculated using the analysis of variance (ANOVA) test for normal continuous variables and the chi-square test for categorical variables.
To investigate the effect of hypothetical factors on infertility in males, we used logistic regression in 3 models: Unadjusted, age- and BMI-adjusted, and fully adjusted models.
The results of unadjusted models showed that TC had a statistically significant effect on infertility (OR, 1.01; 95% CI, 1.00 - 1.01; P = 0.04; Table 2). In other words, according to this model, a 1 mg/dL increase in TC increases the risk of male infertility by 1%. According to unadjusted models, the effect of other variables on infertility was not significant. Based on the age- and BMI-adjusted models, TC (OR, 1.01; 95% CI, 1.01 - 1.01; P = 0.03) and HC (OR, 1.06; 95% CI, 1.00 - 1.12; P = 0.02) were positively related to the risk of infertility occurrence. In model 2, which adjusted for age and BMI, a 1 mg/dL increase in TC increases the risk of male infertility by 1%, and a 1 cm increase in HC increases the risk of male infertility by 6%. The results of the fully adjusted model showed that HC was positively related to the risk of infertility in males (OR, 1.06; 95% CI, 1.00 - 1.13; P = 0.032; Table 2).
| Variables | Model 1 OR (95% CI) a | Model 2 OR (95% CI) b | Model 3 OR (95% CI) c |
|---|---|---|---|
| Age (y; reference: < 40) | |||
| 40 - 50 | 1.07 (0.63 - 1.82) | - | 1.15 (0.66 - 2.02) |
| > 50 | 0.83 (0.62 - 1.12) | - | 0.87 (0.63 - 1.20) |
| BMI (kg/m2) | 0.99 (0.94 - 1.04) | - | 0.91 (0.78 - 1.05) |
| WC (cm) | 1.00 (0.98 - 1.01) | 1.01 (0.97 - 1.05) | 0.99 (0.95 - 1.04) |
| HC (cm) | 1.01 (0.98 - 1.03) | 1.06 (1.00 - 1.12) | 1.06 (1.00 - 1.13) |
| SBP (mmHg) | 1.00 (0.99 - 1.01) | 1.00 (0.99 - 1.02) | 1.01 (0.99 - 1.02) |
| DBP (mmHg) | 1.00 (0.98 - 1.02) | 1.00 (0.98 - 1.02) | 0.99 (0.97 - 1.02) |
| FPG (mg/dL) | 0.99 (0.99 - 1.00) | 0.99 (0.99 - 1.00) | 0.99 (0.99 - 1.00) |
| TC (mg/dL) | 1.00 (1.00 - 1.01) | 1.00 (1.00 - 1.01) | 1.01 (0.98 - 1.05) |
| TG (mg/dL) | 1.00 (0.99 - 1.00) | 1.00 (0.99 - 1.00) | 0.99 (0.99 - 1.00) |
| HDL-C (mg/dL) | 0.99 (0.97 - 1.02) | 0.99 (0.97 - 1.02) | 0.98 (0.94 - 1.02) |
| LDL-C (mg/dL) | 1.00 (0.99 - 1.01) | 1.00 (0.99 - 1.01) | 0.98 (0.95 - 1.02) |
| Educational level (y; reference: < 6) | |||
| 6 - 12 | 2.00 (0.26 - 14.93) | 2.06 (0.27 - 15.51) | 1.33 (0.16 - 10.53) |
| > 12 | 1.75 (0.22 - 13.46) | 1.77 (0.22 - 13.72) | 1.10 (0.13 - 9.04) |
| Smoking history (reference: No) | 0.98 (0.65 - 1.48) | 0.99 (0.65 - 1.51) | 0.94 (0.61 - 1.44) |
| Diabetes mellitus (reference: No) | 0.83 (0.49 - 1.39) | 0.86 (0.50 - 1.47) | 1.04 (0.51 - 2.09) |
| MetS (reference: No) | 0.97 (0.64 - 1.48) | 1.02 (0.64 - 1.61) | 1.12 (0.63 - 2.01) |
| Dyslipidemia (reference: No) | 0.61 (0.35 - 1.07) | 0.61 (0.33 - 1.12) | 0.52 (0.26 - 1.03) |
| CVD (reference: No) | 0.54 (0.27 - 1.06) | 0.54 (0.27 - 1.09) | 0.62 (0.30 - 1.27) |
| Obesity (reference: Underweight) | |||
| Normal weight | 0.51 (0.14 - 1.81) | 0.47 (0.12 - 1.85) | 0.49 (0.12 - 1.99) |
| Overweight | 0.53 (0.15 - 1.83) | 0.45 (0.09 - 2.19) | 0.48 (0.09 - 2.47) |
| Obese | 0.50 (0.13 - 1.80) | 0.38 (0.05 - 2.73) | 0.43 (0.05 - 3.30) |
Abbreviations: BMI, body mass index; WC, waist circumference; HC, hip circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MetS, metabolic syndrome; CVD, cardiovascular disease.
a Model 1: Unadjusted model.
b Model 2: Adjusted for age and BMI.
c Model 3 (fully adjusted): Adjusted for variables in model 2 plus WC, HC, SBP, DBP, FPG, TC, TG, HDL, LDL, educational level, smoking history, diabetes mellitus, MetS, dyslipidemia, CVD, obesity, normal weight, overweight, and obese.
5. Discussion
This study presents the prevalence of self-reported male infertility and metabolic disturbance. The prevalence of self-reported infertility was 6.42%. Moreover, regarding its determinant factors, we found that a 1 unit increase in TC and HC increased the risk of male infertility by 1% and 6%, respectively.
Infertility is a common problem with serious socioeconomic and health implications for individuals and society (29, 30). The results from a global burden of disease study demonstrated that the prevalence of male infertility from 1990 to 2017 increased by 8.2% (30). Also, other investigations found a greater frequency of male infertility (31-33). A recent study indicated a connection between men's overall health and infertility (34, 35).
In a meta-analysis of Iranian studies, the prevalence of male infertility was reported as 2% (11). According to Agarwal et al., 2.5% to 12% of infertility cases are due to the male factor (6). As demonstrated by Krausz and Riera-Escamilla, male infertility is a widespread problem affecting at least 7% of males worldwide and is frequently assumed to have a hereditary predisposition (36). Although several studies have been reported on the prevalence of infertility among couples, little information was available about the prevalence of male infertility in different regions. Apart from this, the methodological assessment of infertility affects the estimated male infertility prevalence rates in different regions. Since we used the self-reported history of infertility, there is a possibility of recall bias, which is common in epidemiology and medical studies (37). However, in Iran, due to sociocultural dimensions of infertility, the possibility of recall bias might decrease. Jung et al. reported that the accuracy of self-reported infertility history was moderate almost 20 years later (38). Another study also reported that self-reported assisted conception had a sensitivity of 83% (39).
Among lipid profiles, only a positive association between infertility and serum TC levels was demonstrated in this study. Although TG and LDL levels were higher in the infertile group, this measure was not significant. Male infertility should be considered as a window to health (40). Eisenberg et al. reported that males with infertility are at higher risk of developing diabetes and ischemic heart disease (41). Another study also reported that childless men had an increased risk of death from CVD (42). Ergun et al. demonstrated that increased LDL and TG had an adverse impact on seminal parameters (43). Also, Hagiuda et al. found that among Japanese patients, the serum TG level was linked to sperm morphological traits (44). It is well-documented that hyperlipidemia might affect the male reproductive system by the alteration in hormone levels, semen parameters, and male reproductive organs (45). It is possible that due to the cross-sectional design, we were unable to demonstrate a causal link between some lipid risk variables and infertility. It is necessary to conduct long-term prospective studies to examine these causal links.
Moreover, in this study, among all obesity-related parameters, there was only a slight positive association between HC and self-reported male infertility. Hip circumference risk may result from its link to MetS (46). However, there is a contradiction in any association between MetS and male infertility (21, 47-49). In our study, there were no significant differences in terms of metabolic abnormalities (MetS and diabetes) between the 2 groups; in addition, these factors were not significant determinants of male infertility in this study.
It is universally acknowledged that obesity could affect human fertility. Evidence showed that the likelihood of subfertility increased by 1.2 times for every 3 kg/m2 increase in BMI, according to a separate study conducted in the United States (50). A recent study indicated that the prevalence of subfertility and infertility was 20% higher among obese individuals (51). The obesogenic environment per se induced OS and inflammatory pathways, which can disrupt the reproductive function (52). Also, Keszthelyi et al. conducted a study on 1169 men who visited an andrology clinic in Budapest for infertility, showing that BMI and waist-to-hip ratio were significantly correlated in all semen parameters (53). Le et al. evaluated 534 men from infertile couples and found that the waist-to-hip ratio was associated with abnormal semen parameters (54). Nonetheless, it should be emphasized that the consequences of male obesity on fertility are likely multidimensional. In addition, obesity commonly coexists with metabolic disorders that enhance the risk of male infertility, such as MetS, hyperlipidemia, CVD, and pro-inflammatory state (55).
Moreover, the findings of our investigation failed to find any link between smoking and male infertility. There is an inconsistency between studies regarding the association between smoking and infertility. Some evidence showed no significant association between male infertility and smoking (56, 57), while others found a positive association between smoking and male infertility (58). Smoking has been shown to negatively impact semen parameters. However, this is still debatable (59, 60). Overall, it is well-documented that all smokers are not infertile (61).
There are some limitations and strengths to this study. The estimated self-reported male infertility is among population-based TLGSs generalized to the urban population. In terms of limitations, this study could not fully cover all risk factors of male infertility due to the lack of measurement of all factors. In this study, the reported male infertility was based on the self-reported questionnaire, and male infertility was not defined by semen data. The absence of this analysis is, therefore, another limitation. Furthermore, this is a cross-sectional study that may be susceptible to problems in a distinction between cause and effect; thus, the results must be judged cautiously. There is a tremendous need to further investigate all causes of male infertility.
5.1. Conclusions
The prevalence of self-reported male infertility was 6.42%. Infertility in males had a positive association with TC and HC, indicating that knowledge about these risks might assist health care professionals and governments in developing and executing measures to change the status quo. In this study, we restrict our attention to males to close the knowledge gap in the infertile male population. However, there are currently little and conflicting epidemiological data. To verify these results, it is necessary to do more research with larger sample sizes.