1. Background
Cardiovascular disease (CVD) is the main cause of death among women, accounting for 35% of all deaths (1). Women experience their first coronary heart disease (CHD) about nine years later than men (2). Also, the risk of CHD is lower in women than in men (30% vs. 50%) at age 40 (3). According to a meta-analysis of the cohort, case-control, and cross-sectional studies, early menopause in women increases the risk of CHD by about 50% (4). In addition, according to population-based cohort studies, surgical menopause increases cardio-metabolic disturbances and the risk of CVD more than natural menopause (5, 6). These studies indicate that estrogen deficiency is related to a higher incidence of CHD, but the causal mechanisms have not been fully assumed.
The favorable effect of estradiol in heart tissue is partly mediated by nitric oxide (NO) production. Additionally, estradiol increases serum levels of NO metabolites (NOx) in post-menopausal women (7) and NO production in the cardiovascular system (8). Estrogen deficiency in post-menopausal women (7) and in ovariectomized (OVX) rats (9) is associated with NO deficiency, which increases the myocardial ischemia-reperfusion (M/IR) injury (10). In animals, ovariectomy frequently increases the inducible NO synthase (iNOS) and decreases the endothelial NOS (eNOS) expression in heart tissue (11-13). Reduced eNOS and neural NOS (nNOS) (14, 15) and increased iNOS expression (16) exacerbate M/IR injury, whereas the increased eNOS (17) and reduced iNOS (18) expression decreases M/IR injury in animal studies.
Detrimental effects of ovariectomy on tolerance to M/IR injury in rats at short-term (2 (19) to 12 weeks (20)) have been reported. However, the effect of ovariectomy in the long term has not been reported previously. Different effects of short- and long-term ovariectomy on heart function (21, 22) and iNOS and eNOS expression and NOx in heart tissue (11, 13) have been reported in experimental studies. We previously reported that long-term ovariectomy (11 months) in rats impairs baseline cardiac function that is correlated with decreased NOx concentration in heart tissue (13); however, this association has not been addressed following IR.
2. Objectives
This study aimed to measure the long-term (11 months) effects of ovariectomy on resistance to M/IR injury in rats and determine whether all three NOS isoforms affect this outcome.
3. Methods
3.1. Animals and Ovariectomized Model Induction
This study used 12 female Wistar rats (6-month-old weighting 200 - 220 g). Rats were housed in polypropylene cages (42 × 28 × 15 cm, 3 rats in each cage) under standard conditions of 12-h light (7 am to 7 pm) and 12-h dark (7 pm to 7 am), at 21 ± 2°C, with free access to tap water and regular food. All experiments followed the published guidelines for the care and use of laboratory animals in Iran (23) and were reported following ARRIVE guidelines (24). The care and use of rats were confirmed by the Research Institute for Endocrine Sciences (RIES) Ethics Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.ENDOCRINE.REC.1401.115). In accordance with the principle of the 3Rs (reduce, refine, replace), we obtained isolated hearts for our experimental interventional study from a previous study. This allowed us to reduce the number of animals used in our research. The previous study investigated the effects of nitrate administration on carbohydrate metabolism in OVX rats (25). Therefore, details on the induction and verification of the OVX model have been described earlier (25). In brief, to induce the OVX model, two dorsolateral skin incision methods were used to remove ovaries from anesthetized rats (sodium pentobarbital at a dose of 60 mg/kg). After surgery, to reduce the risk of self-mutilation and infection of the skin after suturing, the rats were housed individually for 7 days, and penicillin was added to the surgery place to prevent infection (26).
To verify the model, body weight was measured before and after two months of ovariectomy, and blood samples were taken from the tail tips of OVX rats under isoflurane inhalation anesthesia. In addition, 17β-estradiol, progesterone, luteinizing hormone (LH), and Follicle-stimulating Hormone (FSH) concentrations in serum were measured at the start of the study and 2 months after ovariectomy. Intra-assay Coefficients of Variation (CVs) for all assays were < 7%. Kits for measurements of estradiol (Cat. No. CAN-E-430, sensitivity 10 pg/mL) and progesterone (Cat. No. CAN-P-305; sensitivity 0.1 ng/mL) were obtained from Diagnostics Biochem Company (Ontario, Canada) and for LH (Rat LH ELISA kit, Cat. No. CSB-E12654r; sensitivity 0.15 mIU/mL) and FSH (Rat FSH ELISA kit, Cat. No. CSB-E06869r; sensitivity 0.07 mIU/mL) were obtained from Cusabio Biotech (Wuhan, China) company. The success rate of induction of the OVX model was 100% in the current study.
3.2. Experimental Design
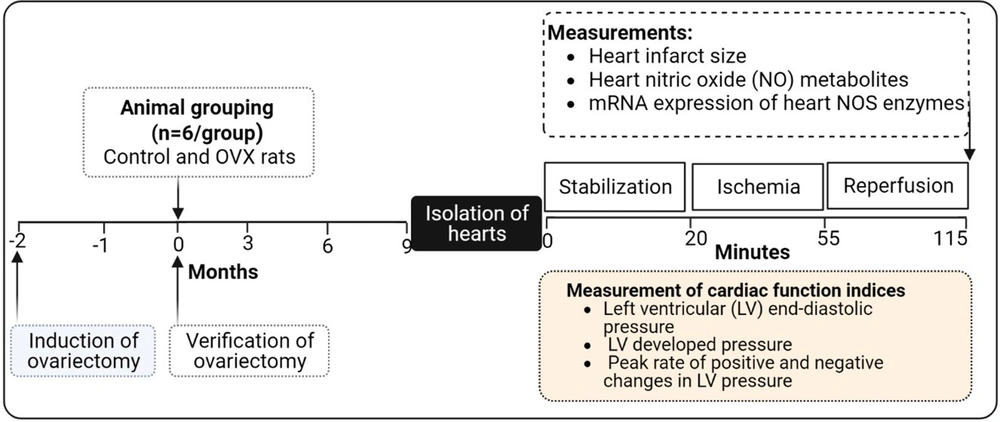
The experimental design is presented in Figure 1. After confirming the model, rats were divided into 2 groups: Control and OVX (n = 6 in each group). At the end of the study (11 months after ovariectomy), hearts of the anesthetized rats in control and OVX groups were isolated and exposed to a 35 min global ischemia and 60 min reperfusion in the Langendorff apparatus. Cardiac function indices (CFIs) were recorded during the experiment. At the end of the reperfusion period, the hearts were used to measure infarct size, levels of NOx, and mRNA expression of eNOS, nNOS, and iNOS.
3.3. Preparation of Ischemia-Reperfusion Injury Model in Langendorff-perfused Rat Hearts
For the preparation of the IR injury model, rats in control and OVX groups were anesthetized with an intraperitoneal injection of sodium pentobarbital at a dose of 60 mg/kg, and the hearts were immediately isolated at the end of the study, immersed in ice-cold perfusion buffer, the aorta cannulated and connected to the Langendorff apparatus. Retrograde perfusion was performed with Krebs-Henseleit solution (KHS), with a composition of 2.5 mM CaCl2, 118.6 mM NaCl, 1.6 mM MgSO4, 1.2 mM KH2PO4, 4.7 mM KCl, 25 mM NaHCO3, and 11.1 mM glucose (all from Merck, Darmstadt, Germany), equilibrated with 95% O2:5% CO2 (pH 7.40). Isolated hearts from all rats were subjected to 20 min of stabilization to obtain baseline hemodynamic parameters and, subsequently, exposed to 30 min of global ischemia and 60 min of reperfusion. Therefore, the IR injury model in Langendorff-perfused rat hearts was induced by blocking the total retrograde perfusion to the heart for 30 min and subsequent reperfusion for 60 min.
3.4. Assessment of Hemodynamic Parameters
For the measurement of CFI, including left ventricular end-diastolic pressure (LVEDP), LV developed pressure (LVDP), and peak rate of positive (+dp/dt) and negative (-dp/dt) changes in LV pressure during stabilization (20 min), global ischemia (35 min), and reperfusion (60 min) periods, a latex balloon was inserted into the left ventricle, and LVEDP was adjusted at 5-10 mm Hg in all hearts by filling water in the latex balloon. Also, LVEDP, LVDP, and ± dp/dt were digitalized by a data acquisition system (Power Lab, AD instrument, Australia).
3.5. Assessment of Heart Nitric Oxide Metabolites
At the end of the study, heart tissues (0.1 g) were homogenized in 0.5 mL phosphate-buffered saline. After centrifuging 10,000 g for 10 min, the modified Griess method measured NOx in all homogenates. To deproteinize homogenates, zinc sulfate (10 µL, 15 mg/mL) and NaOH (10 µL, 3.72 M) were added to each homogenate, and supernatants were centrifuged at 10,000 g for 10 min. To measure NOx concentrations, 0.1 mL of vanadium trichloride (8 mg/mL in 1 M HCl) and then 0.05 mL N-(1-naphthyl) ethylenediamine (0.1% in ddH2O) and 0.05 mL of sulfanilamide (2% in 5% HCl) were added to the deproteinize homogenates. Samples were then incubated for 30 min at 37°C, and the optical density was read at 540 nm. Nitrite was measured similarly, except that HCl (1 M) was added to the samples to replace vanadium trichloride. The nitrate concentration was determined by subtracting nitrite from NOx concentrations in all samples. The Bradford method measured the protein concentration in the samples, and NOx levels were reported per mg protein. Intra-assay CVs of NOx and nitrite in heart tissue were 2.8% and 3.3%, respectively.
3.6. Measurement of Infarct Size
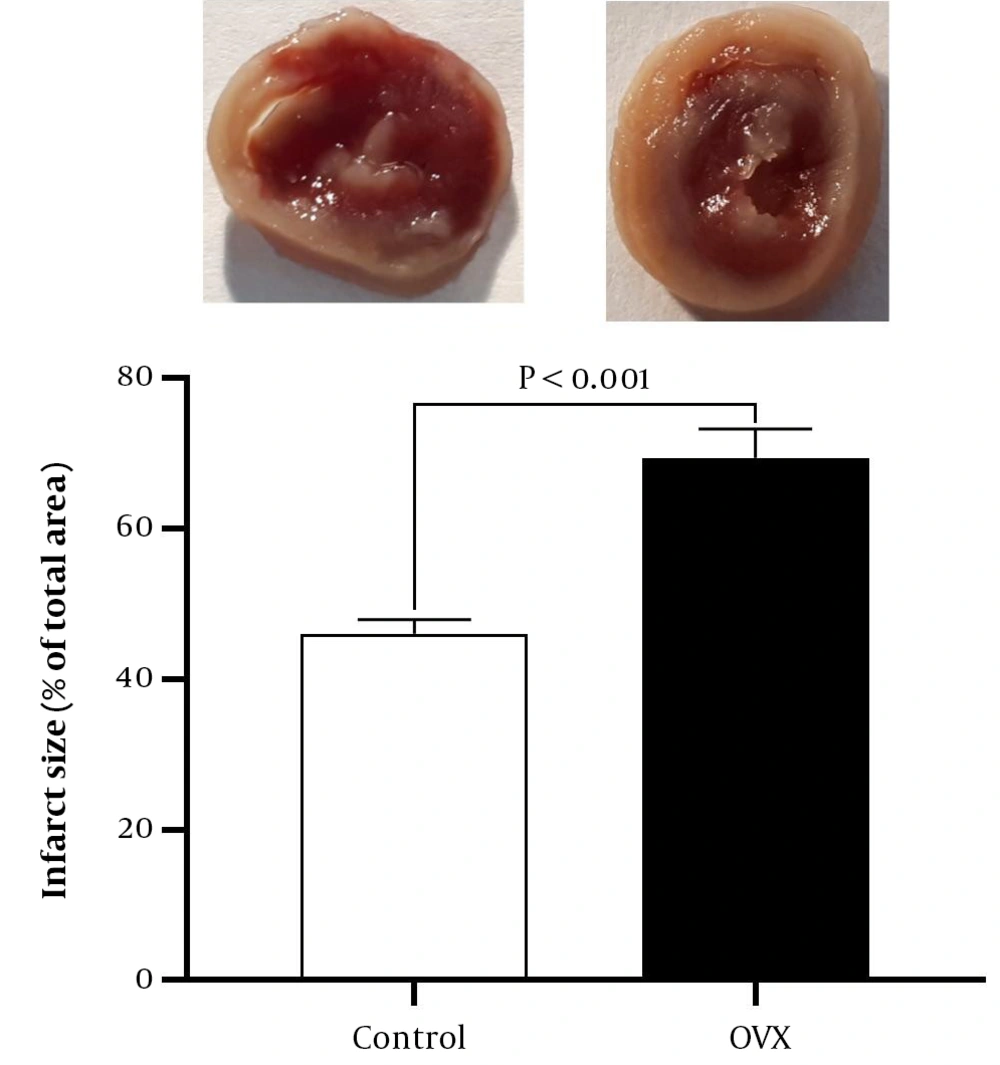
The Triphenyl Tetrazolium Chloride (TTC) method was used to measure infarct size in isolated hearts. In brief, the iced hearts were sliced thinly, incubated in TTC (1% in phosphate buffer solution, 37°C for 10 min), and fixed in formalin (10% for one day) to detect viability from the necrotic areas. The sections were photographed, analyzed by Image J software, and represented as a percentage of the total area.
3.7. Measurement of mRNA Expression
The primer sequences of the target and reference genes are presented in Table 1. The TRIzol reagent (Invitrogen, USA) was used for RNA extraction from hearts. To synthesize and amplify cDNA, the cDNA synthesis kit (SMOBiO Technology, Taiwan) and Amplicon SYBR Green Master Mix in a real-time PCR machine (Rotor-Gene 6000, Corbett, Life Science, Australia) were used, respectively.
| Name | Sequence (5´-3´) | Gene Bank Accession No. | Size of Product (bp) |
|---|---|---|---|
| Neuronal NOS | NM_052799 | 126 | |
| F | AATCTCAGGTCGGCCATCAC | ||
| R | ATCCCCCAAGGTAGAGCCAT | ||
| Endothelial NOS | NM_021838.2 | 100 | |
| F | TGACCCTCACCGATACAACA | ||
| R | CGGGTGTCTAGATCCATGC | ||
| Inducible NOS | NM_012611 | 93 | |
| F | TGGCCTCCCTCTGGAAAGA | ||
| R | GGTGGTCCATGATGGTCACAT | ||
| ß-actin | NM_031144.3 | 100 | |
| F | CGTCCACCTGCTAGTACAAC | ||
| R | CGACGACTAGCTCAGCGATA |
Abbreviation: NOS, nitric oxide synthase.
3.8. Statistical Analyses
Data analysis was done by the GraphPad Prism software (Version 8), except for mRNA expressions, which are reported as relative fold changes using the REST software. The paired t-test was used to compare the serum estradiol and progesterone concentrations, LH and FSH, heart NO metabolites, and infarct size. To compare the CFI in control and OVX rats at different time points of reperfusion, two-way mixed (between-within) ANOVA was used, followed by the Bonferroni post-hoc test. Relative expressions of NOS enzymes were analyzed based on their cycle thresholds versus ß-actin using the REST software. Two-sided P-values < 0.05 were considered statistically significant.
4. Results
4.1. Verification of Model
Two months after ovariectomy, OVX rats, at month 0, had significantly (P < 0.001) higher body weights by 21% (248.5 ± 6.2 vs. 205.0 ± 2.5 g) and lower serum concentrations of progesterone and estradiol by 76% (13.2 ± 2.6 vs. 56.8 ± 8.4 ng/mL) and 91% (8.2 ± 1.3 vs. 91.4 ± 16.7 pg/mL), respectively. In addition, OVX rats, at month 0, had significantly higher (P < 0.001) serum concentrations of LH by 38.6 folds (57.9 ± 15.7 vs. 1.5 ± 0.3 mIU/mL) and FSH by 6.3 folds (266.0 ± 70.7 vs. 42.4 ± 4.8 mIU/mL).
4.2. Response to Myocardial Ischemia-Reperfusion Injury
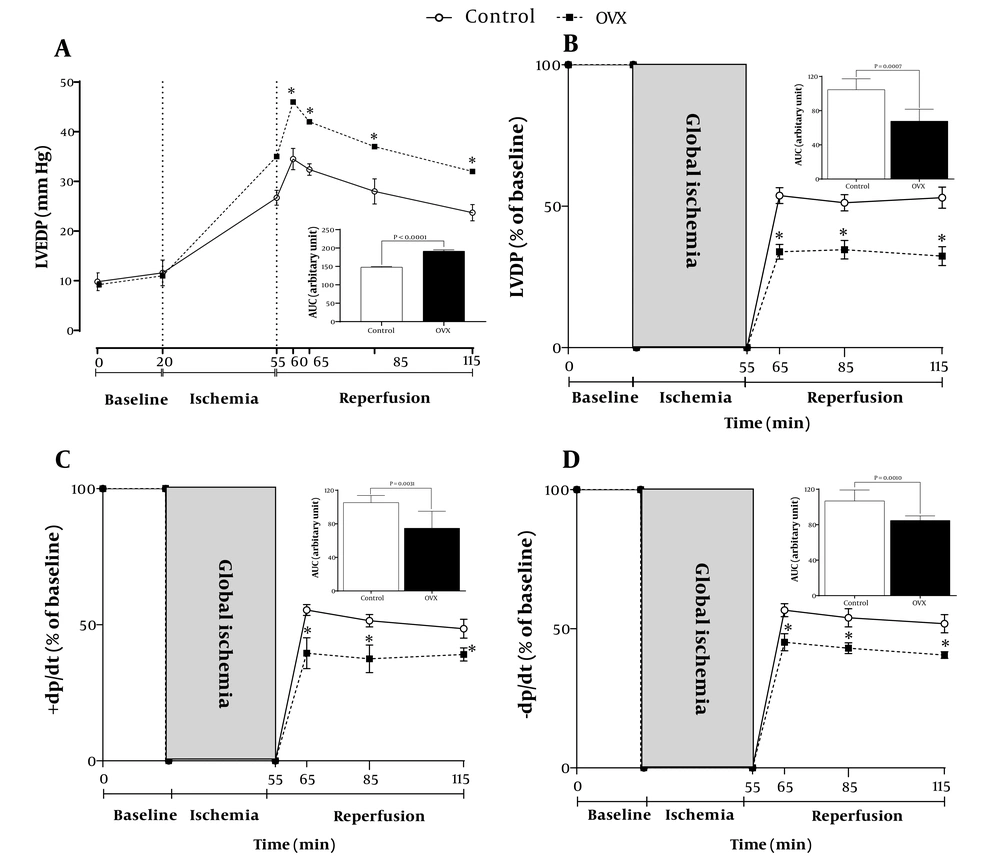
Isolated hearts from OVX rats showed higher sensitivity to M/IR injury (P < 0.001), as indicated by increased LVEDP by 29% and decreased recovery of +dp/dt, –dp/dt, and LVDP by 29%, 22%, and 35%, respectively (Figure 2). In addition, according to Figure 3, OVX rats had significantly larger (P < 0.001) infarct size by 51% at the end of the experiment.
4.3. Nitric Oxide Metabolites Concentration and Nitric Oxide Synthase Expression
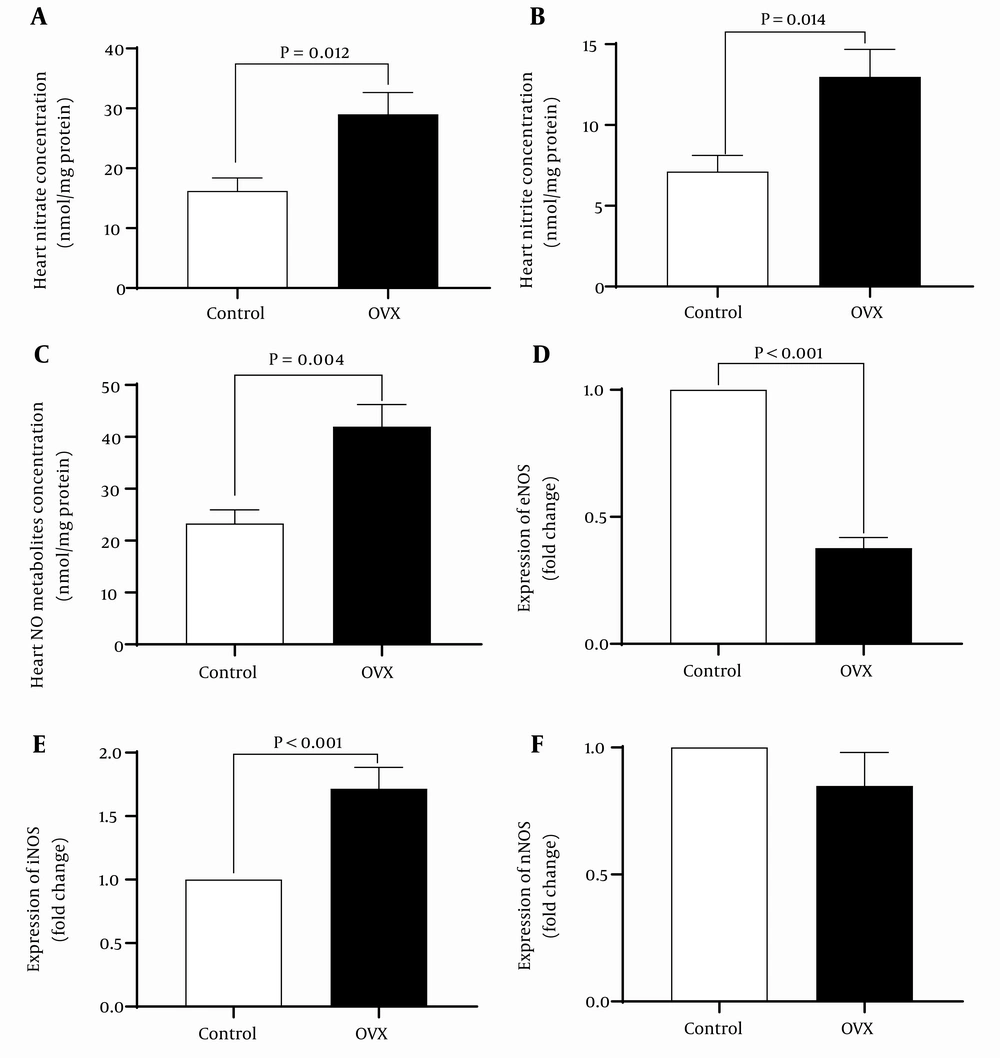
As shown in Figure 4, isolated hearts from OVX rats at the end of reperfusion had significantly higher (P < 0.001) nitrate, nitrite, and NOx concentration by 79%, 82%, and 83%, respectively. These changes in NOx levels are associated with reduced mRNA levels of eNOS by 38% and increased mRNA levels of iNOS by 71%. In addition, long-term ovariectomy had no effect on mRNA levels of nNOS following IR.
5. Discussion
The current study showed the detrimental effects of long-term ovariectomy against M/IR injury as characterized by the reduced recovery of LVDP, ± dp/dt, and increased LVEDP and infarct size. These detrimental effects of long-term ovariectomy are associated with decreasing eNOS and increasing iNOS expression in heart tissue.
In the current study, two months after ovariectomy, serum concentrations of estradiol and progesterone decreased, and LH and FSH increased, all confirming the induction of the OVX rat model (26). In addition, in OVX rats, we observed a bodyweight increase that aligns with previous reports (26). After ovariectomy, estrogen deficiency increases body weight by increasing visceral obesity (27), redistributes body fats from the peripheral to the abdominal regions (28), increasing adipocyte size (25), and decreasing energy expenditure without changing food intake (29, 30).
In this study, hearts from OVX rats showed less tolerance against M/IR injury, as indicated by the reduced recovery of LVDP, ± dp/dt, and increased LVEDP. To the best of our knowledge, no studies have reported the effects of long-term OVX on CFI following exposure to ischemia. Previous studies have shown the detrimental short-term (2 (19), 3 (31), 4 (32), 5 (32), 6 (33), and 12 weeks (20)) effects of ovariectomy against M/IR injury in rats. In addition, similar to our results, increased infarct size by 45-75% have been reported 4 (32), 5 (21), and 12 (20) weeks after OVX. Our data extend this effect to 11 months in OVX rats. It has been reported that estrogen deficiency exacerbates response to M/IR injury, at least partly by increasing myocardial oxidative stress (34). In support of this, studies have reported that levels of malondialdehyde and reactive oxygen species increased while levels of reduced glutathione decreased in the heart tissue of OVX rats after exposure to ischemia (20, 21).
In the current study, hearts from OVX rats had significantly higher nitrate, nitrite, and NOx concentrations by 79%, 82%, and 83%, respectively. In line with our study, NOx levels in the coronary effluent of rats, as an indicator of NO production in isolated hearts, increased in the OVX rat after 40 days of ovariectomy (35). In addition, our results showed that long-term ovariectomy decreased eNOS by 38% and increased iNOS by 71%, while it did not affect mRNA levels of nNOS following IR. In line with our results, lower eNOS (21), higher iNOS (21, 35), and unchanged nNOS (35) have been reported in OVX rats. Inconsistent with our results, unchanged iNOS and eNOS expressions 4 (11), 8 (12), and 7 (35) weeks after surgery have been reported in the heart tissue of OVX rats. This disagreement might be because of the different periods of OVX. It has been reported that OVX did not affect iNOS and eNOS levels after 6 - 8 weeks (11, 12, 35); however, a major change was detected after 4 weeks (21, 35). Estradiol increased NO bioavailability in the cardiovascular system by increasing mRNA and protein expression of eNOS (5). It has been reported that increased expression and activity of eNOS in the aorta of hypertensive rats have been observed after estradiol receptors activation (6). In addition, after menopause, estradiol's antioxidant activity decreased, further decreasing NO bioavailability in the cardiovascular system (7).
Nitric oxide is recognized as a double-edged sword in heart tissue. It is attributed to eNOS at low levels and has protective effects against M/IR, while at high levels, it is attributed to iNOS and has detrimental effects against M/IR (36, 37). During ischemia, NO in the heart is predominantly derived from iNOS; thus, its inhibition (18) protects, whereas its overexpression exacerbates M/IR injury (16, 38). Also, iNOS-derived NO contributes to M/IR injury by reducing eNOS expression (39) and increasing peroxynitrite formation (40). In addition, the downregulation of eNOS exacerbates the heart's response to ischemia (41). Therefore, decreased NO bioavailability and increased oxidative stress could impair response to M/IR injury in OVX rats.
5.1. Limitations, Strengths, and Suggestions
As the strengths of the present work, we evaluated the contribution of all NOS enzymes in response to M/IR injury in OVX rats. Furthermore, the OVX rat model used in the present study shows menopause features observed in women after surgical menopause (26). This study has some limitations. It did not address possible mechanisms responsible for the changes in NOS enzyme expressions in OVX rats; several parameters may affect it, including decreased sex hormones and blood pressure. Also, pharmacological interventions were not used to check the contribution of NOS enzymes to the detrimental effects of ovariectomy on cardiac function in rats. Finally, we used the isolated heart in the Langendorff apparatus that does not fully reflect hormonal and neuronal regulation affecting the heart's response to IR injury (42).
5.2. Conclusions
Long-term estrogen deficiency increased iNOS expression and decreased eNOS expression in the heart tissue of OVX rats. Imbalanced NOS expressions were related to deteriorated responses to M/IR injury in OVX rats.