1. Introduction
Graves’ disease (GD), the most common cause of hyperthyroidism, is an autoimmune condition. There are several definitive treatments for GD, such as antithyroid drugs (ATDs), radioiodine therapy (RAI), and thyroidectomy. Antithyroid drugs are the first-line treatment in many cases because they are easy to administer, less invasive, and deliver a high remission rate. These medications can also be used as an adjunctive treatment to achieve a euthyroid state before RAI or thyroidectomy to prevent thyroiditis or thyroid storm during the procedure. However, the administration of ATDs carries the risk of hepatotoxicity (1), so an alternative therapy is needed when ATD administration is impossible. One novel bridging treatment in this condition is therapeutic plasma exchange (TPE) to substitute the plasma volume with a replacement fluid. This therapeutic modality is usually used for various diseases, especially hematologic conditions, and is expected to also reduce the thyroid hormone level in plasma by removing plasma with apheresis, followed by the infusion of replacement fluid (2). However, the use of TPE in hyperthyroid conditions is still limited. In this case presentation, we describe the successful use of TPE as a bridging therapy to achieve a euthyroid state before thyroidectomy.
2. Case Presentation
A 35-year-old man was admitted to the emergency room due to general weakness, complaining of headaches, palpitation, chest discomfort, nausea, fever, weight loss, and tremor. The patient declared a history of fatty liver disease and elevated liver transaminases. The history of testing for viral hepatitis markers was negative. He reported no history of smoking, hypertension, cardiac problems, and kidney disease. There was a history of thyroid cancer in her mother.
On initial assessment, the patient was fully alert; his blood pressure was 154/90 mmHg; he had a regular pulse (130 beats/minute), a respiration rate of 18 times/minute, and a body temperature of 37.2°C, and peripheral oxygen was 97% on room air. Clinical examination revealed lid retraction, Goiter with no bruit, enlarged liver, hyperreflexia, and tremor. There were no gastrointestinal or central nervous system manifestations. The electrocardiogram showed sinus tachycardia. Laboratory tests revealed white blood cells (WBC) = 4.5 × 109/L, hemoglobin = 125 g/L, HDL cholesterol = 25 mg/dL, triglyceride = 195 mg/dL, ALT = 123 U/L, TSH = 0.005 µIU/mL, and FT4 = 7.77 ng/dL (Table 1, day 0). Markers for viral hepatitis (A, B, C, and E) were non-reactive. Ultrasonography of the thyroid gland showed enlargement of the right lobe, left lobe, isthmus, and increased thyroid parenchymal vascularization. Abdominal ultrasonographic examination revealed mild hepatomegaly with mild fat infiltration. Other sonographic findings included a normal-sized gall bladder, no thickening of the gall bladder wall, no biliary ductal dilatation, and no cholelithiasis. The patient was diagnosed with GD with a suspected thyroid storm and elevated liver transaminases due to fatty liver disease. The patient daily received 20 mg of methimazole, 120 mg of oral propranolol (divided into two doses), and 80 mg of glycyrrhizin (intravenously). The patient was admitted to the General ward to closely monitor his clinical condition.
During observation, the patient developed anorexia, fever (39.4°C), hypotension (blood pressure: 79/52 mmHg), and tachycardia of 120 beats/minute. Laboratory findings showed increased liver transaminases and bilirubin levels (Table 1, day 6). The patient was diagnosed with a thyroid storm and transferred to the intensive care unit. The patient had an intravenous fluid infusion, received dobutamine intravenously, 300 mg of intravenous hydrocortisone (daily, divided in three doses), 120 mg of glycyrrhizin (intravenous, daily), and 750 mg of ursodeoxycholic acid (oral, daily, divided in three doses). Methimazole was also continued. On day 9th of hospitalization, the fever subsided, and vital signs were within the normal range. However, icteric signs worsened, and laboratory findings showed a significant increase in liver transaminases and bilirubin levels (Table 1, day 9). The patient was diagnosed with suspected impending liver failure due to drug-induced liver injury. Therefore, methimazole and hydrocortisone were stopped since there were no signs of thyroid storm. On day 11th of hospitalization, the patient was transferred to the general ward, on day 15th of hospitalization, the patient was discharged from the hospital as his clinical condition improved.
During the follow-up period (days 19 - 26), thyrotoxicosis symptoms returned along with an increase in thyroxine levels after the discontinuation of ATDs. Radioactive iodine uptake (RAIU) was 28.1%, and the patient was prepared for total thyroidectomy as a definitive treatment.
Since the patient had a history of methimazole-induced liver injury, ATD was stopped. Therefore, TPE was initially performed to reduce thyroxine levels. The patient was admitted again for TPE and preoperative preparations. Albumin 5% served as the replacement fluid. The plasma volume exchanged was 2700 - 3000 mL/session. The patient daily received 3 grams (three ampules) of calcium gluconate intravenously given in three doses along with daily 5000 IU vitamin D3 peroral to prevent hypocalcemia.
After five sessions of TPE, thyroxine and antibody levels substantially decreased (Table 1, days 37 - 46). During the preoperative period, the patient daily received 60 mg of oral propranolol (divided into three doses), Lugol’s solution (oral, 15 drops given in 3 doses from five days before the surgery), and 60 mg of hydrocortisone peroral (given in 3 doses from three days before surgery).
| General Ward | Intensive Care Unit | General Ward | Outpatient Clinic | General Ward | Normal Range | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 ATD Start | Day 2 | Day 4 | Day 6 | Day 9 ATD Stop | Day 11 | Day 13 | Day 15 | Day 19 | Day 21 | Day 26 | Day 37 After 1st TPE | Day 39 After 2nd TPE | Day 41 After 3rd TPE | Day 43 After 4th TPE | Day 46 After 5th TPE | Day 47 | Day 48 Surgery Day | ||
| Complete Blood Count | |||||||||||||||||||
| HB, g/L | 125 | 121 | 112 | 112 | 0 | 114 | 116 | 0 | 0 | 0 | 0 | 124 | 118 | 119 | 113 | 125 | 111 | 130 - 180 | |
| HCT, % | 39 | 37 | 34 | 35 | 35 | 36 | 37.6 | 35.8 | 36.2 | 34.7 | 38.5 | 33.7 | 40.0 - 48.0 | ||||||
| PLT, ×109/L | 234 | 224 | 150 | 113 | 179 | 201 | 162 | 174 | 163 | 190 | 214 | 321 | 150 - 400 | ||||||
| WBC, ×109/L | 4.5 | 7.9 | 3.1 | 2.27 | 1.94 | 7.5 | 5.6 | 5.6 | 8.1 | 6.9 | 9.1 | 5.0 - 10.0 | |||||||
| DIFF | |||||||||||||||||||
| NEUT, % | 79 | 81 | 95 | 77 | 69 | 58.2 | 61 | 73.6 | 50.0 - 70.0 | ||||||||||
| LYMPH, % | 8 | 11 | 2 | 10 | 12 | 23.6 | 23.6 | 13.6 | 20.0 - 40.0 | ||||||||||
| MONO, % | 11 | 8 | 3 | 11 | 16 | 13.3 | 11.4 | 10.8 | 22.0 - 8.0 | ||||||||||
| EOS, % | 2 | 0 | 0 | 1 | 2 | 4.7 | 3.8 | 1.9 | 1.0 - 3.0 | ||||||||||
| BASO, % | 0.2 | 0.2 | 0.1 | 0.0 - 1.0 | |||||||||||||||
| Liver Functional Tests | |||||||||||||||||||
| ALT, U/L | 123 | 184 | 339 | 1023 | 562 | 272 | 166 | 150 | 114 | 124 | 57 | 42 | 35 | 39 | 105 | < 35 | |||
| AST, U/L | 296 | 128 | 84 | 47 | 34 | 54 | 51 | 36 | 31 | 30 | 28 | 24 | 84 | < 40 | |||||
| Total bilirubin, µmol/L | 23.94 | 99.18 | 258.21 | 90.63 | 59.90 | 46.17 | 37.62 | 37.79 | 32.15 | 19.49 | 11.80 | 10.09 | < 17.1 | ||||||
| Direct bilirubin, µmol/L | 10.26 | 68.4 | 164.16 | 58.14 | 35.91 | 27.36 | 20.52 | 23.60 | 20.18 | 12.65 | 8.55 | 4.79 | < 4.3 | ||||||
| Indirect bilirubin, µmol/L | 30.78 | 94.05 | 32.49 | 23.94 | 18.81 | 14.19 | 11.97 | 6.84 | 3.25 | 5.30 | < 12.8 | ||||||||
| Thyroid Markers | |||||||||||||||||||
| TSH, µIU/mL | 0.005 | 0.311 | 0.005 | < 0.003 | 0.001 | 0.001 | 0.002 | 0.002 | 0.002 | 0.002 | < 0.003 | < 0.003 | 0.27 - 4.7 | ||||||
| FT4, ng/dL | 7.77 | 3.64 | 4.38 | 3.64 | 3.3 | 3.02 | 2.69 | 2.33 | 1.91 | 1.24 | 1.07 | 1.1 | 0.8 - 2.0 | ||||||
| Total T3, nmol/L | 8.86 | > 9.21 | 0.98 - 2.34 | ||||||||||||||||
| TRAb, IU/L | 9.90 | 1.77 | 1.39 | < 1.75 | |||||||||||||||
| Anti-TPO, IU/mL | 29.16 | 6.89 | 7.70 | < 8 | |||||||||||||||
Abbreviations: ATD, antithyroid drug; TPE, therapeutic plasma exchange; HB, hemoglobin; HCT, hematocrit; PLT, platelet; WBC, white blood cells; DIFF, differential blood count; NEUT, neutrophils; LYMP, lymphocytes; MONO, monocytes, EOS, eosinophils; BASO, basophils; ALT, alanine transaminase; AST, aspartate transamianase; TSH, thyroid stimulating hormone; FT4, free thyroxine 4; TRAb, thyroid hormone receptor antibody; TPO, thyroid peroxidase.
On the day of the surgery, the dose of hydrocortisone was changed to 200 mg daily (given in 2 intravenous doses until three days after surgery). There was no sign of adrenal crisis, and tapering off was performed. The patient was discharged from the hospital five days after the surgery with the medications CaCO3 (1500 mg daily, given in 3 doses), calcitriol (0.25 mcg daily), and vitamin D3 (5000 IU daily). Levothyroxine (50 mcg daily) was started ten days after the surgery when the thyroid hormone started to decline.
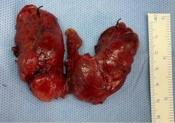
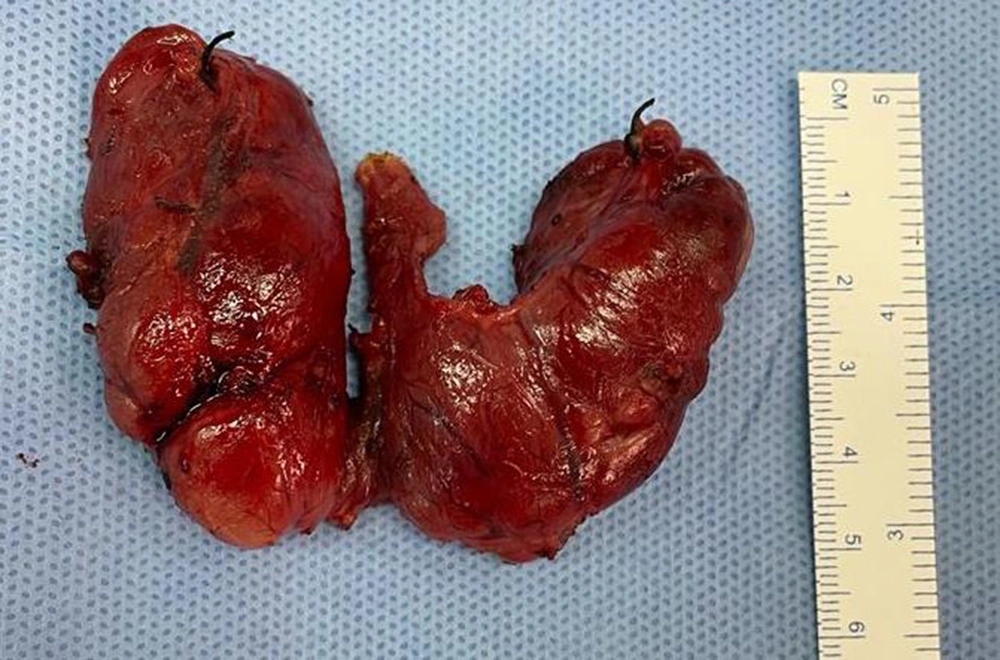
Thyroid histopathology showed follicles of varying sizes lined with simple cuboidal to columnar epithelium. Parts of the epithelium were hyperplastic and invaginated as papillary folds into the lumen. The lumen contained colloids, some of which had a "scalloping" appearance. Local epithelial cells with vesicular nuclei and a "clearing" appearance were noticed. Moderate infiltration by inflammatory cells was observed in the local stroma, and areas with congestive blood vessels and fibrosis were evident (Figure 1).
3. Discussion
3.1. Grave’s Disease
This is an autoimmune disease caused by the anti-thyroid-stimulating hormone receptor (TSH-R) autoantibodies produced by autoreactive B cell clones, activating TSH-R and inducing the production of thyroid hormones. Grave’s disease is more common in females, with a female: male ratio of 10:1. The inactivation of one of the X chromosomes of females seems to be responsible for the predominance of this condition among women. However, in this case, the patient was a male with a history of thyroid disease in his family (his mother’s thyroid cancer), supporting the theory that considers a role for genetic factors, especially HLA genes, in the risk of GD (3, 4).
Regardless of the patient’s gender, GD symptoms vary widely depending on the severity of thyrotoxicosis and individual susceptibility to excessive release of thyroid hormones. During the clinical examination of GD patients, the physician may encounter a history of weight loss, palpitation, tremor, the presence of diffuse Goiter, pretibial myxedema, and ophthalmopathy. Grave’s disease is usually diagnosed by measuring TSH, free thyroxine levels, and TSH-R autoantibodies (4). In our patient, initial assessments showed a decrease in TSH and increased FT4 (beyond three folds of the upper limit normal (ULN)) and TRAb levels. Thyroid histopathological examination was also consistent with GD.
3.2. Thyroid Storm
Thyroid storm, an unusual yet life-threatening complication of hyperthyroidism, is hyperthyroidism with multiple organ decompensation triggered by physical or mental stress. The diagnosis of thyroid storm can be confirmed using the Burch-Wartofsky Point Scale (BWPS) or Japan Thyroid Association criteria (JTA) (5, 6). On day 6th after admission, our patient’s BWPS score was 55, which was highly suggestive of thyroid storm, and based on JTA criteria, the patient was definitely diagnosed with this condition. Therefore, he was managed as suggested for treating a thyroid storm and received 300 mg intravenous hydrocortisone daily divided into three doses. Unlike BWPS, JTA criteria mandate the presence of thyrotoxicosis to diagnose thyroid storm to minimize false positive results.
3.3. Hyperthyroidism-Induced Liver Abnormality
In untreated conditions, the prevalence of hyperthyroidism accompanied by at least one abnormal liver functional test is about one in two and even higher in GD patients (about two-thirds) (7). Hyperthyroidism increases the body’s metabolic activity and the liver’s oxygen demand, resulting in hepatic ischemia and infarction. Besides, excessive amounts of triiodothyronine induce apoptosis in hepatocytes. Liver abnormalities are more prevalent in GD patients than in people suffering from other hyperthyroidism conditions, which is attributed to TRAb-triggered hepatic inflammation. It has been suggested that liver abnormality in untreated hyperthyroidism patients can be either caused by direct toxicity against hepatocytes or secondary to its complication, such as heart failure (8). In such conditions, liver dysfunction is resolved after treatment with methimazole. Consistently, our patient showed elevated liver transaminases on the initial assessment before treatment; however, fatty liver disease could have also contributed to the condition.
3.4. Graves' Disease Management
The primary goals of GD treatment are to control hyperthyroidism symptoms and achieve long-term remission. Therapeutic modalities for GD are ATD (thionamides), radioactive iodine (RAI) therapy, and thyroidectomy. Thionamides, such as propylthiouracil, carbimazole, and methimazole, are the first-line treatment in some regions because they are inexpensive and easy to be administered in a less-invasive manner. The remission rate after ATD treatment has been 63.5% (9). The predictors of remission after treatment with ATD include female gender, being a non-smoker, lower initial serum TRAb levels, and duration of treatment of more than 24 months (10). Thus, our patient had a low chance of achieving remission with ATD treatment since he was a male with a high level of TRAbs.
Methimazole should be administered for nearly all patients receiving ATD therapy, except for patients who are in the first trimester of pregnancy, diagnosed with a thyroid storm, and have had poor responses to methimazole and refused RAI therapy or surgery. Initial methimazole daily dosages are as follows: 5-10 mg for FT4 levels 1 - 1.5 times the ULN, 10 - 20 mg for FT4 levels 1.5 - 2 times the ULN, and 30 - 40 mg for FT4 levels 2 - 3 times the ULN. Leukocyte counts with differentials and liver functional tests, including transaminases and bilirubin levels, should be determined before initiating ATD since agranulocytosis and hepatotoxicity are among the potential side effects of this therapeutic approach (1). Our patient received 20 mg methimazole daily because he showed elevated liver transaminases even though the FT4 levels more than three times the ULN on the initial assessment.
3.5. Methimazole-Induced Liver Injury
In our patient, after nine days of receiving 20 mg methimazole daily, the patient’s icteric appearance worsened, and liver transaminases continued to rise, followed by an increase in bilirubin levels without a clear sign of intrahepatic cholestasis or hepatobiliary obstruction. The patient was diagnosed with suspected impending liver failure due to mixed-type drug-induced liver injury (DILI). Despite having no sign of liver failure, such as encephalopathy, coagulopathy, or edema, we suspected the diagnosis of impending liver failure because of significant fluctuations in liver transaminases and bilirubin levels. It is noteworthy that methimazole is known to cause idiosyncratic DILI, which can be triggered suddenly with unpredictable effects and varying severity upon varying time intervals (11, 12). Some mechanisms have been proposed for ATD-induced liver injury, suggesting a role for n-methyl thiourea and glyoxal as metabolites involved in methimazole-induced liver injury (11). The management of DILI relies on two options based on its pathophysiology. In the dose-dependent type, the dosage of the suspected drug should be adjusted, and in the idiosyncratic type, the drug should be discontinued. Methimazole is categorized as a drug associated with the idiosyncratic type (11), so we withdrew methimazole, which led to an improvement in the patient’s liver transaminases and bilirubin levels; however, thyrotoxicosis symptoms returned.
3.6. Plasma Exchange as Bridging Therapy for Definitive Treatment
Radioactive iodine therapy is an option for patients with contraindications for or inability to achieve euthyroid in response to ATD treatment or those with comorbidities that may aggravate by surgery. A 5-year investigation found a transient increase in the first year and then a slight decrease in the following year in TRAb levels after RAI therapy compared to pharmaceutical treatment and surgical management, indicating a higher autoimmune activity after RAI therapy, which can lead to a lower incidence of remission (13). Hence, it is not advisable to choose RAI therapy for a patient who needs rapid remission.
Finally, we decided to perform a thyroidectomy for this patient. In a study, the success rates for achieving the euthyroid state were 2.33 and 94.45 times higher for subtotal and total thyroidectomy than RAI (14). A combination of β-adrenergic blockade, potassium iodide, glucocorticoids, and cholestyramine can be administered as adjunctive treatment before surgery (1). Here, our patient received Lugol’s solution, hydrocortisone, and propranolol prior to surgery.
The patient must be in euthyroid condition before surgery to prevent thyroid storm. As we could not administer ATD, alternative therapeutic options were sought for this purpose, and TPE was chosen. This extracorporeal process removes a large volume of plasma and replaces it with a substitute fluid. Centrifugal TPE (cTPE) shows higher plasma removal efficiency and shorter procedure time than membrane TPE (mTPE). However, cTPE uses citrate as an anticoagulant, which potentially increases the risk of hypocalcemia (2). In our patient, prophylactic gluconate as calcium was used to prevent and treat citrate-induced adverse effects and surgery complications, which was effective, and no episode of hypocalcemia occurred.
During TPE, about 60 - 70% of substances in plasma are eliminated per each 1 - 1.5 exchanged volume. In this process, T3 and T4, which are firmly bound to plasma proteins, are also removed and replaced with fluids. Moreover, pathologic antibodies, immune complexes, cytokines, and coagulation factors are eliminated, and coagulation factors achieve their pre-TPE levels within 4 - 24 hours. Albumin, as a replacement fluid, has shown a relatively large capacity but a low-binding affinity for thyroid hormones, lowering the concentration of free thyroid hormones. Several plasma exchange procedures can be performed to achieve clinical improvement (2, 15). Our patient underwent five sessions of TPE, with a total plasma exchange volume of 2700 - 3000 mL per session, which was replaced with albumin of 5%. The reduction in FT4 level per each TPE session was 8 - 25%, with a total decrease of 62%, and total reductions in TRAbs and anti-TPO Abs were 86% and 74%, respectively. Finally, two days after the last TPE (to allow the recovery of the normal coagulative state), the patient underwent total thyroidectomy and developed no complications, such as bleeding, adrenal crisis, or thyroid storm, during the perioperative period.
3.7. Conclusions
Definitive therapy is advisable for GD patients who cannot tolerate ATD. Surgery is recommended rather than RAI therapy in individuals with a history of severe DILI caused by ATD. This is because surgery carries a lower risk of developing thyroid storm and a shorter time to achieve remission compared to RAI therapy, requiring ATD, which the patient cannot tolerate. We here reported that TPE was a safe and effective bridging therapy to achieve the euthyroid state before surgery.