1. Introduction
Papillary thyroid carcinoma (PTC) is the most common endocrine malignancy, accounting for approximately 85% of all differentiated thyroid cancers derived from follicular cells. It has a 10-year survival of around 93% and is considered an indolent tumor (1). However, a small group of PTC variants (i.e., diffuse sclerosing, tall cell, columnar cell, solid, and hobnail) has more aggressive behavior, with higher rates of recurrence and metastasis, and in some cases, with loss of the iodine-131 (I-131) uptake and lower survival rates (2). In these cases, treatment with tyrosine kinase inhibitors (TKIs) might be necessary (3).
Lenvatinib, one of the TKIs approved for PTC, works by inhibiting vascular endothelial growth factor (VEGF) receptors and platelet-derived growth factor receptors, which help block tumor angiogenesis and impede tumor growth (4). Treatment with this molecule might prolong the survival of patients with inoperable thyroid cancer (5, 6). However, lenvatinib has been associated with multiple side effects, including hypertension, bleeding, arterial and venous thrombotic embolism, hepatopathy, nephropathy, perforation of tissues compromised by the tumor, gastrointestinal fistula, posterior reversible encephalopathy syndrome (PRES), cardiac disorders, hand-foot syndrome, myelosuppression, hypocalcemia, delayed wound healing, diarrhea, and fatigue (7).
Posterior reversible encephalopathy syndrome is a clinical and radiological entity first described in 1996 by Hinchey et al. in a series of 15 patients with acute neurological symptoms, including headache, seizures, visual disturbances, and other focal neurological deficits (8). The clinical presentation is varied, ranging from symptoms such as headache and confusion to focal deficits and seizures. Imaging findings are typical, involving cortical-subcortical regions bilaterally in the parietal and occipital regions with vasogenic edema (9, 10). The edema associated with PRES is a consequence of the failure of cerebral blood flow autoregulation, which explains the altered mental status and other neurological symptoms. This phenomenon is produced by rapid changes in cerebral perfusion pressure or by a systemic inflammatory response leading to the leakage of intravascular fluid and the production of edema (11).
Posterior reversible encephalopathy syndrome can develop due to hypertension or the use of immunosuppressants, cytotoxic agents, and specific-target molecules. However, the exact cause is still unknown (11). In the last few decades, its recognition has improved due to the increased availability of magnetic resonance imaging (MRI).
Three radiological patterns of PRES have been described based on the location and extent of white matter involvement, which is evidenced in brain MRI as hyperintensities in T2 and fluid-attenuated inversion recovery (FLAIR) sequences (11), a parieto-occipital pattern, a hemispheric watershed pattern with the involvement of the borderline areas between the anterior cerebral and the middle cerebral arteries with manifestations in the frontal, parietal and occipital lobes, and an atypical pattern, with the involvement of other regions, including the cerebellum and brainstem, also associated with parieto-occipital changes.
Treatment is based on supportive measures and symptomatic management. For patients with elevated blood pressure, a 25% reduction in mean arterial pressure is recommended within the first few hours. If it is possible to identify the triggering drug, it should be discontinued (10). Patients with seizures might require a loading dose of antiepileptic drugs, and patients with severely altered mental status might require intubation to secure the airway (12).
With the introduction of new targeted cancer therapies, this syndrome has become a side effect observed with TKIs; however, it is still rare (13). This case report describes a patient with iodine-refractory metastatic PTC who developed PRES associated with using lenvatinib.
2. Case Presentation
A 65-year-old woman with a history of arterial hypertension, obesity, normocalcemic hyperparathyroidism without bone or kidney implication, and depressive disorder was diagnosed with PTC at the age of 51 years. The initial treatment involved total thyroidectomy, total bilateral neck dissection, and ablation therapy with 150 millicuries (mCi) of I-131. Post-therapeutic radioactive iodine (RAI) whole-body scan (WBS) showed no evidence of metastatic radioiodine avidity. The initial staging was T4aN1bM0.
During follow-up, dynamic risk stratification for cancer recurrence had a persistently incomplete biochemical response given by elevated thyroglobulin levels from the initial treatment despite adequate thyroid-stimulating hormone (TSH) suppression. In the 6th year of follow-up, pulmonary metastases were identified. The patient was then treated with two RAI therapy of 200 mCi each over the next 2 years (cumulative I-131 dose of 550 mCI). After the last session, the RAI WBS showed that pulmonary lesions lost the ability for iodine uptake, and 18F-fluorodeoxyglucose positron emission tomography-computed tomography scans (18F-FDG PET-CT) revealed an increase in the number, size, and metabolic activity of the pulmonary lesions, besides the appearance of a metabolically active bone lesion in the right iliac (Table 1).
| Date (m-y) | TSH (uIU/mL) | Tg (ng/dL) | TgAb (IU/mL) Reference: 0 - 4.11 |
|---|---|---|---|
| 2008 - 2013 | No available data on laboratory tests | ||
| 06 - 2014 | 0.27 | 40.42 | 2.26 |
| 02 - 2016 | PET: Pulmonary nodules increasing in number with metabolic activity | ||
| 11 - 2016 | 3.24 | 152.56 | 1.82 |
| 03 - 2017 | 163.75 | 1.49 | |
| 03 - 2017 | Started sorafenib 800 mg daily | ||
| 02 - 2018 | PET: Decrease in size and activity of pulmonary nodules | ||
| 08 - 2018 | Sorafenib 400 mg daily | ||
| 12 - 2018 | 0.65 | 166.78 | 1.80 |
| 06 - 2019 | 0.04 | 259.8 | 0.2 |
| 07 - 2019 | PET: Increase in number and metabolic activity of pulmonary nodules - new mediastinal and cervical lesions | ||
| 07 - 2019 | Sorafenib 600-800 mg daily | ||
| 06 - 2020 | 0.05 | 772.6 | 1.05 |
| 09 - 2020 | PET: Increase in number and metabolic activity of pulmonary nodules | ||
| 05 - 2021 | 0.03 | 752.3 | 1.59 |
| 06 - 2021 | PET: Increase in number and metabolic activity of pulmonary nodules and mediastinal lesions | ||
| 11 - 2021 | Malignant pleural effusion | ||
| 02 - 2022 | PET: Increase in number and metabolic activity of pulmonary nodules and mediastinal lesions | ||
| 03 - 2022 | Started lenvatinib 24 mg daily | ||
| 04 - 2022 | Posterior reversible encephalopathy syndrome | ||
| Stopped lenvatinib | |||
| 05 - 2022 | Restarted lenvatinib 10 mg daily | ||
| 08 - 2022 | 0.02 | 169 | 1.11 |
| 08 - 2022 | Lenvatinib 14 mg daily | ||
Abbreviation: PET, 8F-fluorodeoxyglucose positron emission tomography-computed tomography scan; TSH, thyroid-stimulating hormone; Tg, thyroglobulin; TgAb, thyroglobulin autoantibodies.
aThyroglobulin autoantibodies were measured by ARCHITECT® Anti-Tg Chemiluminescent Microparticle Immunoassay for the quantitative determination of the immunoglobulin G (IgG) class of TgAb in the human serum.
Given the refractoriness to iodine, sorafenib 800 mg per day was initiated, which resulted in stable disease of the pulmonary lesions at the 6 months 18F-FDG PET-CT control. Nonetheless, the dose had to be reduced to 400 mg per day due to hand-foot syndrome and diarrhea. One year later, 18F-FDG PET-CT revealed an increase in the number and metabolic activity of pulmonary lesions and the appearance of new mediastinal and cervical lesions. Additionally, the thyroglobulin levels markedly increased (from 166 to 259.8 ng/dL in 6 months) with negative anti-thyroglobulin antibodies, and TSH was adequately suppressed (Table 1).
Physicians decided to switch the treatment to lenvatinib 24 mg per day; however, this was impossible due to difficulties with the patient’s health insurance. She continued to take sorafenib for 2 more years at doses within 600 - 800 mg per day according to her tolerance. Over time, thyroglobulin levels continued to increase (up to 700 ng/dL), and pulmonary lesions progressed, eventually triggering a left malignant pleural effusion that required decortication, pleurodesis, and due to a later septic collection, surgical reintervention with the use of vacuum-assisted closure system for 3 months.
One month after discharge, she was started on lenvatinib 24 mg per day, and 4 weeks later, she was brought by an ambulance to the emergency department with an intense holocranial headache preceded by three episodes of a few minutes of duration characterized by loss of consciousness, tonic upward eyeballs deviation, and tonic-clonic movements of the arms that became generalized. Ambulance personnel reported normal blood pressure within the events; nevertheless, on admission, she had a blood pressure of 183/110 mmHg, heart rate of 120 beats per minute, respiratory rate of 18 breaths per minute, oxygen saturation of 90% at room air, and finger stick glucose level of 119 mg/dL.
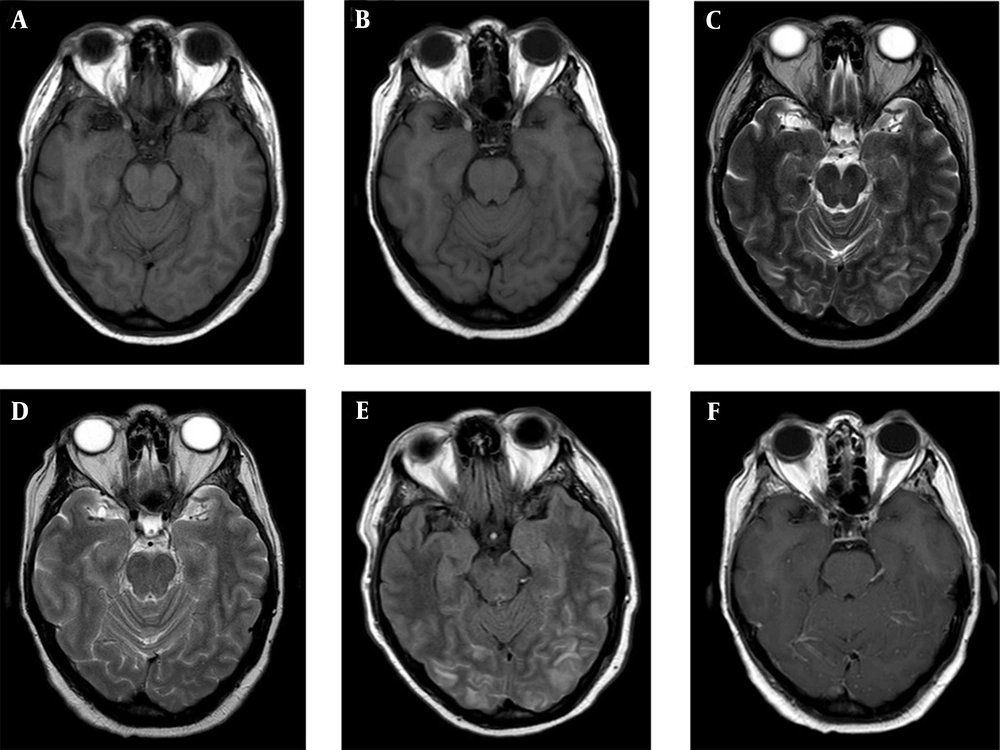
She was in a somnolence state with no other alterations upon physical examination. Levetiracetam 3.000 mg was intravenously administrated, and a simple head computed tomography showed no bleeding, ischemic areas, or tumors. A brain MRI was performed, which showed cortico-subcortical hyperintensities predominantly in the posterior parieto-occipital region, followed by the periventricular white matter, splenium callosum, and nuclei basal region, without representation in the diffusion, suggestive of PRES (Figure 1). Lenvatinib was discontinued, and anti-hypertensive management was adjusted, with the total normalization of blood pressure after 7 days. The patient required hospitalization for 2 more weeks due to difficulty controlling her headache, which was finally resolved with a pericranial blockade. No measurements of thyroglobulin levels or anti-thyroglobulin antibodies were taken during hospitalization.
At her follow-up visit 4 weeks later, she remained asymptomatic, with controlled blood pressure, and no seizure or headache was present. She had a normal physical examination, prompting the restarting of lenvatinib at a daily dose of 10 mg, with no adverse reactions (Table 1). Three months after the follow-up visit, the patient was still asymptomatic, with a decline in thyroglobulin levels (169 ng/dL) and ambulatory blood pressure monitoring within the normal range. The contrasted brain MRI control showed the disappearance of the occipitoparietal, pericallosal, subcortical, and nucleo-basal hyperintensities without areas of malacia or gliosis, suggesting the resolution of PRES (Figure 1). The dosage of lenvatinib was increased to 14 mg per day to evaluate the tolerance of the drug at the next appointment, which is pending at the time of writing this case report.
3. Discussion
Herein, we present the case of a patient with a history of high-risk PTC who, during her disease course, developed pulmonary and bone metastases and met the criteria for iodine refractoriness. As a result, she required the use of TKIs, starting with sorafenib. Due to the progression of the lesions and the inability to reach maximum doses due to intolerable adverse effects, lenvatinib was used for second-line management, which ultimately led to PRES.
Sorafenib and lenvatinib are the two multitargeted ITKs, approved by the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA) as a first-line therapy for patients with RAI-refractory locally advanced or metastatic differentiated thyroid cancer. There is no head-to-head randomized clinical trial comparing these drugs. However, in the phase III trial of each one compared to a placebo, sorafenib was reported with 10.8 versus 5.8 months in the placebo group of median progression-free survival, with a hazard ratio (HR) of 0.59 (95% confidence interval (CI): 0.45 - 0.76, P < 0.0001) (14), and lenvatinib was reported with 18.3 versus 3.6 months in the placebo group with an HR of 0.21 (99% CI: 0.14 - 0.31, P < 0.0001) (6). An important methodological design difference between the sorafenib and lenvatinib trials is that patients who received previous targeted therapy or chemotherapy for thyroid cancer were excluded from the sorafenib trial. Nevertheless, the patients in the lenvatinib trial could have previously received those treatments. This was the case with the patient in the present report. A recent retrospective cohort study showed longer progression-free survival with lenvatinib (35.3 months) than sorafenib (13.3 months) with a statistical significance (P = 0.001) as the first-line therapy for patients with RAI-refractory differentiated or poorly differentiated thyroid cancer (15).
Although the complete pathophysiology of PRES has not been elucidated, some conditions that predispose to its development are known, including poorly controlled arterial hypertension, vascular diseases, kidney disease, sepsis, and the consumption of some medications, especially immunosuppressants. The association between drugs targeting receptors that regulate vascular permeability and PRES is increasing (11), which has led to case reports of specific molecular target drugs, including TKIs, such as cediranib, sunitinib, sorafenib, pazopanib, and regorafenib (13, 16-19).
As mentioned above, the trial that led to the FDA approval of lenvatinib for iodine-refractory differentiated thyroid cancer demonstrated increased progression-free survival and increased adverse events, including one case of PRES among 261 patients exposed to the drug (6). To date, only 3 reported cases worldwide have shown the association between the use of lenvatinib in thyroid cancer and the development of PRES (20-22); however, none occurred in a Latin population.
Lenvatinib inhibits VEGF receptors 1 - 3 to disrupt tumor angiogenesis and, therefore, tumor invasion and metastasis. The vascular endothelial growth factor regulates vasomotor tone and maintains blood pressure by dilating arterioles and small venules (23). As a systemic drug, lenvatinib might impact cerebral blood pressure regulation and contribute to the development of PRES.
In the present case, the patient experienced symptoms and images typical of PRES. The suspension of lenvatinib treatment and symptomatic management allowed the patient to control blood pressure and the absence of new seizure episodes. Additionally, the patient’s MRI showed the resolution of white matter lesions in the following months. This finding suggests that treatment with lenvatinib was the probable cause of PRES. After the reintroduction of the drug at lower doses, the patient did not present any neurological symptoms, and blood pressure remained under control with established antihypertensive drug therapy.
3.1. Conclusions
With the advent of innovative therapies, new adverse effects manifest themselves. Therefore, the use of TKIs in inoperable thyroid cancer and/or refractory to I-131 can cause serious events, of which healthcare personnel and patients should be aware to control the risk factors associated with their development and identify them quickly. This case report, the first in South America, provides information on the development of PRES with the administration of lenvatinib in a Hispanic population and raises awareness to consider the monitoring and optimal management of hypertension every time the drug is used.