1. Background
Urbanization phenomenon with industrialization has led to epidemiological transition from communicable diseases to chronic diseases (1, 2). Noncommunicable diseases are the main cause of death worldwide (1-3). Cardiovascular disease (CVD) is considered as the main cause of death in most countries with some well-known metabolic risk factors like diabetes, hypertension, and hypercholesterolemia (4). CVD is also the first and most common cause of death in Iran (5). Recently, in a study performed by Talaei et al. on an Iranian population in Isfahan, the mortality rate of CVD was reported to be 331 and 203 per 10 0000 person-years in males and females, respectively (6). CVDs were also found as a cause of more than 40% of deaths in Tehran (7), and the incidence rate of CHD has been 6.5 and 11.9 per 1000 person-years in females and males of this capital city, respectively (8). On the other hand, the prevalence of CHD was 10.7%, 6%, and 11.8% based on Rose Angina Questionnaire (RQ), self-report, and electrocardiogram (ECG) in this city, respectively (9). However, the incidence of recurrent CVD has not yet been reported in Iran.
Early detection of CHD plays an important role in early intervention and reduces future risk of serious disease. There are simple and low cost methods in population-based studies for assessing high risk individuals such as RQ, ie, a standardized instrument for measuring typical angina in population surveys (10), and Minnesota ECG coding , a more objective measure for CHD events in populations (11, 12).
To understand the usefulness and effectiveness of each screening test, the 4 following key points should be noted: (1) the cost of the screening test; (2) the frequency of undesirable detected outcome; (3) the relationship between abundance and survival of diagnosed patients; and (4) the ability to reduce adverse outcomes (morbidity and mortality) by the information obtained from the screening test (13).
2. Objectives
In the present study, we aimed at assessing the roles of ECG and RQ in predicting new CHD events, independent of other cardiovascular risk factors, and assessing the incidence rate of recurrent CHD among people with a positive history. Moreover, we aimed at comparing the predictive power of these measurements with that of diabetes and other metabolic risk factors. More detailed information helps predict CHD events precisely and improves quality of care services (14).
3. Methods
3.1. Study Population
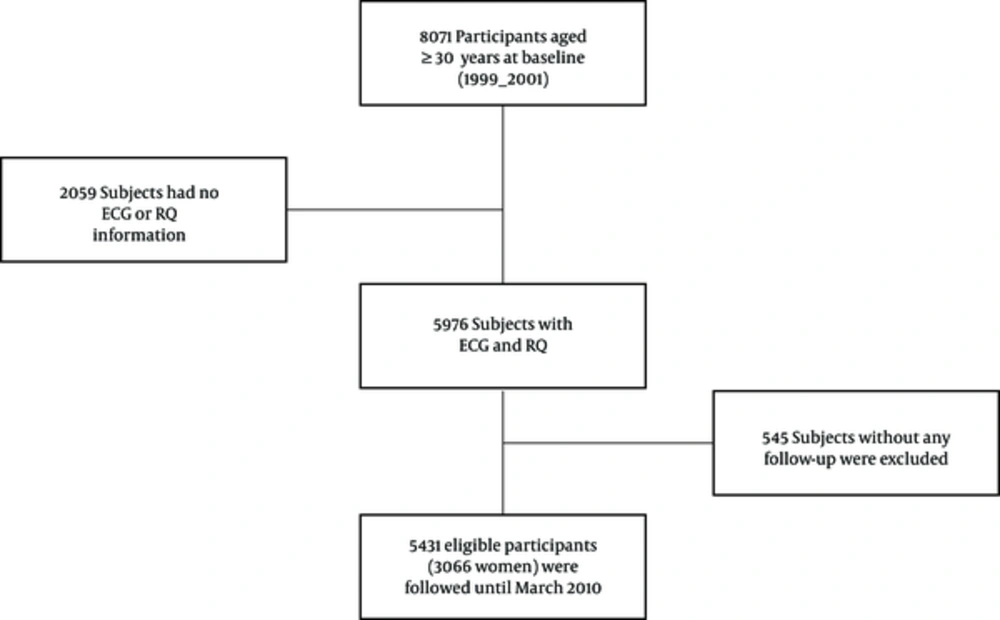
This study was conducted in the framework of Tehran lipid and glucose study (TLGS), which is a population-based cohort study in Tehran with the aim of determining the incidence and prevalence of noncommunicable diseases and their risk factors. This study has been described in details before (15, 16). There were 8071 individuals aged 30 years or older in the first phase of TLGS, of whom 5976 participants completed ECG and RQ. Among the 5976 participants, 545 individuals were excluded due to lack of follow-up, and the remained 5431 participants were followed until March 20, 2010 with a median follow-up of 10.3 years (Figure 1). Informed consent was signed by the all participants, and the study protocol was approved by the ethics committee of research institute for endocrine sciences at Shahid Beheshti University of Medical Sciences.
3.2. Exposure
The participants were categorized into 3 groups according to the Minnesota code and Whitehall criteria: (1) probable CHD (codes of 1.1.1 through 1.1.7, and 1.2.1 through 1.2.8); (2) possible CHD (codes of 1.3.1 through 1.3.6; 4.1.1 through 4.4; 5.1 through 5.3 or 7.1.1 through 7.1.2); (3) and non-CHD (ECGs that had none of these criteria) (17). We defined ECG-positive CHD as probable or possible CHD at baseline. In the study, the Persian version of RQ was used as it is a reliable tool for detecting angina pectoris in Iranian population (18).
At baseline, the participants were classified into 5 categories based on self-reported history of CHD, ECG, and RQ results: 1, History-Rose-ECG: negative CHD history, ECG and Rose (the reference group); 2, History-Rose+ECG-: negative CHD history, negative ECG, and positive Rose; 3, History-Rose-ECG+: negative CHD history, negative Rose, and positive ECG; 4, History-Rose+ECG+: Negative CHD history, positive ECG and positive Rose; and 5, History+: positive self-reported CHD history.
3.3. Outcome
The outcome was defined as the occurrence of CHD or death from CHD. The participants were censored at the time of their last follow-up, death due to any other causes or at the end of study (March 20, 2010), and whichever came first.
In brief, each participant was under continuous follow-up for any CHD event leading to hospitalization or death, confirmed by an outcome committee. Details of the CHD outcome data have been published previously (8). The CHD outcome was comparable with ICD-10 rubric I20-I25.
3.4. Potential Confounders
At the baseline visit (i.e, 1999 - 2001), data were collected using interview, physical examination, and laboratory measurements. Using pretested questionnaires, trained interviewers interviewed the participants. Information on age, gender, medical history of CHD, smoking habit, and family history of CVD were collected. A blood sample was taken after 12 to 14 hours of overnight fasting. The measurements of fasting plasma glucose (FPG), 2-h plasma glucose (2hPG), systolic and diastolic blood pressure (SBP and DBP), HDL cholesterol (HDL-C), and total cholesterol (TC) have been described in detail elsewhere (19). Participants were classified as diabetic if they reported the use of glucose lowering medications, FPG ≥ 7.0 mmol/l (126 mg/dl) or 2h-PG ≥ 11.1mmol/l (200 mg/dl). Hypertension was defined as systolic/diastolic blood pressure equal or higher than 140/90 mm Hg or using hypertension medication; and hypercholesterolemia was defined as serum cholesterol ≥ 6.21 mmol/l (240 mg/dl) or using lipid drugs.
3.5. Statistical Methods
Baseline characteristics of the participants were presented as mean (SD) or frequency (percentage). Kaplan-Meier survival curves were constructed for the 5 study groups and compared using the log-rank test. The hazard ratios with 95% confidence intervals (95% CIs) in groups (2-5) relative to the reference (History-Rose-ECG-) were estimated using the Cox proportional-hazards regression model. Multivariable Cox regression model was used to estimate the hazard ratios (95% CIs) adjusted for the potential confounders mentioned above. To finely adjust for the confounding effect of age, the time origin was set to date of birth, making time-since-birth analysis time. The Cox proportional hazards assumption was visually assessed by log-log plot, ie, the plot of -log-log (S (t)) curves for levels of CHD history against log (t). The interaction between gender and study groups was tested in the analysis and because there was no significant interaction, a pooled regression analysis over gender was conducted to obtain more efficient estimates. Adjusted comparisons between the study groups in pairs were conducted using the multivariable Cox regression models. Using Wald test, the HRs of metabolic risk factors (hypertension, hypercholesterolemia, and diabetes) were compared to the HRs of the study groups. All statistical analyses were conducted using Stata software, Version 11 (Stata Corp, College Station, TX, USA).
4. Results
Generally, 2575 (43.1%) of the 5976 participants were male, of whom 167 (6.5%) had positive history of CHD. Positive history of CHD was detected in 157 (4.6%) of females. Baseline characteristics of the study participants in each level of CHD history are presented in Table 1.
| Variables | With History of CHD (n = 324) | Without History of CHD (n=5652) | P Value | |
|---|---|---|---|---|
| Gender | Female | 157 (48.5) | 3244 (57.4) | 0.002 |
| Diabetes | Yes | 89 (27.5) | 568 (10.05) | 0.001 |
| Hypertension | Yes | 211 (65.1) | 1443 (25.5) | 0.001 |
| Hypercholesterolemia | Yes | 146 (45) | 1655 (29.3) | 0.001 |
| Cigarette smoking | Yes | 108 (33.3) | 1333 (23.6) | 0.001 |
| BMI | 25 - 30 | 149 (46) | 2458 (43.5) | 0.021 |
| ≥ 30 | 102 (31.4) | 1529 (27) | ||
| HDL | < 40 | 173 (53.4) | 2782 (49.2) | 0.14 |
| Age (years); Mean (SD) | 60.3 (9.5) | 47.1 (12.06) | 0.001 | |
Baseline Characteristics by CHD Historya of 5976 Participants in Tehran Lipid and Glucose Study
A total of 562 CHD events occurred during 50 856 person-year of follow-up, of which 320 events were in males (21 626 years of follow-up). As presented in Table 2, CHD incidence rate among all participants was 11.05 cases per 1000 person-year. Incidence rates of CHD in males with and without history of CHD were 62.87 (95% CI: 49.39 - 80.03) and 12.35 (95% CI: 10.92 - 13.96) cases per 1000 person-year, respectively. These figures were 49.26 (95% CI: 37.63 - 64.48) and 6.71 (95% CI: 5.82 - 7.74) in females.
| Variables | Number of New Cases | Incidence Rate (IR) Per 1000 | 95% CI for IR | |
|---|---|---|---|---|
| Lower | Upper | |||
| Male | ||||
| History-Rose-ECG- | 186 | 10.4 | 9.0 | 12.0 |
| History-Rose+ECG- | 27 | 26.0 | 17.8 | 37.8 |
| History-Rose-ECG+ | 33 | 23.5 | 16.7 | 33.1 |
| History-Rose+ECG+ | 8 | 44.6 | 22.3 | 89.1 |
| CHD+ | 66 | 62.9 | 49.4 | 80.0 |
| Female | ||||
| History-Rose-ECG- | 119 | 5.1 | 4.3 | 6.1 |
| History-Rose+ECG- | 29 | 12.6 | 8.8 | 18.2 |
| History-Rose-ECG+ | 34 | 15.2 | 10.8 | 21.2 |
| History-Rose+ECG+ | 7 | 24.4 | 11.6 | 51.2 |
| CHD+ | 53 | 49.3 | 37.6 | 64.5 |
| Both sex | ||||
| History-Rose-ECG- | 305 | 7.4 | 6.6 | 8.2 |
| History-Rose+ECG- | 56 | 16.7 | 12.9 | 21.8 |
| History-Rose-ECG+ | 67 | 18.3 | 14.4 | 23.3 |
| History-Rose+ECG+ | 15 | 32.1 | 19.3 | 53.3 |
| CHD+ | 119 | 55.9 | 46.7 | 67.0 |
| Total | 526 | 11.05 | 10.17 | 12.0 |
Incidence Rate of CHD by the Group Variable of the Study Separately in Male and Female Participants
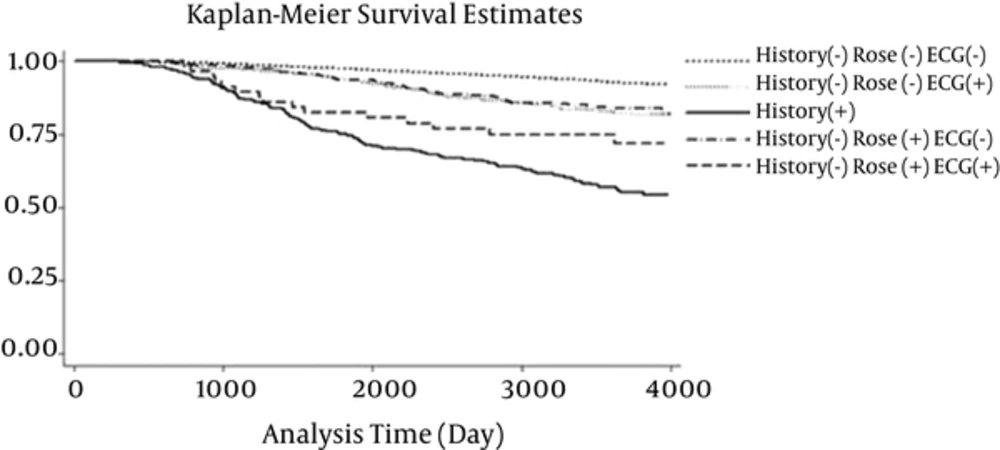
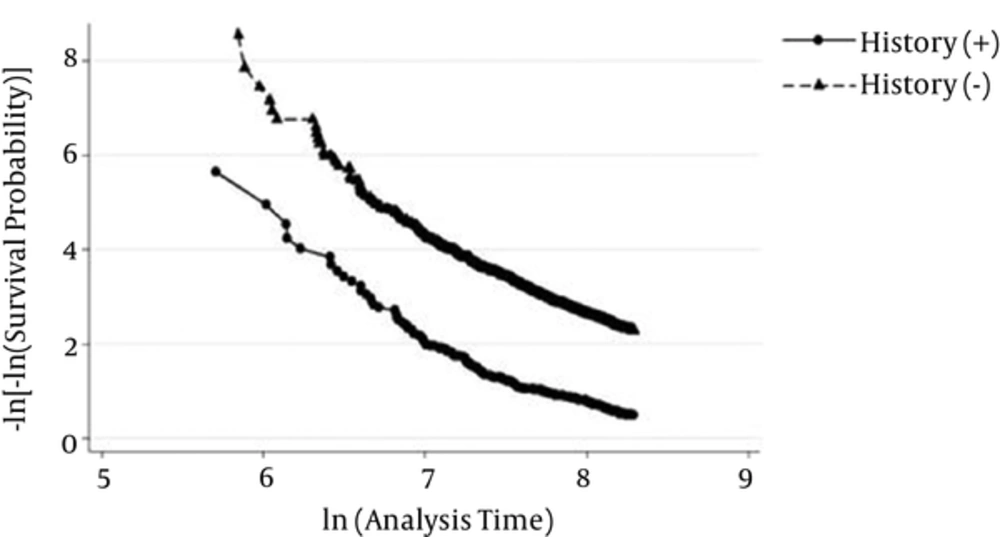
Kaplan-Meier survival curves for the 5 categories are demonstrated in Figure 2. Although a significant difference was shown in general (P < 0.001), the Kaplan Meier survival curves for History-Rose-ECG+ and History-Rose+ECG- were the same. The approximately parallel curves in log-log plot suggest that the Cox proportional hazards assumption was satisfied for the CHD history (Figure 3). The results of the multivariable Cox regression model are presented in Table 3. Adjusting for potential confounders, the hazard ratio of new CHD in participants with CHD history was 4.11 (95% CI: 3.27 - 5.11) times of the reference group. Compared to the reference group, the hazard ratios in participants with positive Rose, positive ECG, and both were 2.18 (95% CI: 1.63 - 2.90), 1.92 (95% CI: 1.47 - 2.51), and 2.48 (95% CI: 1.46 - 4.20), respectively (Table 3). Furthermore, except for BMI and HDL, all confounders had a significant relationship with CHD events.
| Variables | Total (n = 5431) | |
|---|---|---|
| HR (%95 CI) | P Value | |
| Groups (History-Rose-ECG- as reference) | ||
| History-Rose+ECG- | 2.18 (1.63 - 2.90) | < 0.001 |
| History-Rose-ECG+ | 1.92 (1.47 - 2.51) | < 0.001 |
| History-Rose+ECG+ | 2.48 (1.46 - 4.20) | 0.001 |
| CHD+ | 4.11 (3.27 - 5.11) | < 0.001 |
| Gender (male vs. female) | 1.91 (1.56 - 2.34) | < 0.001 |
| Familial history of CVD (yes vs. no) | 1.37 (1.12 - 1.67) | 0.002 |
| Diabetes (yes vs. no) | 3.04 (2.52 - 3.67) | < 0.001 |
| Hypercholesterolemia (yes vs. no) | 1.64 (1.37 - 1.96) | < 0.001 |
| Hypertension (yes vs. no) | 2.10 (1.75 - 2.52) | < 0.001 |
| Cigarette smoking (yes vs. no) | 1.42 (1.16 - 1.73) | < 0.001 |
| HDL (≥ 40 vs. < 40) | 0.85 (0.72 - 1.02) | 0.085 |
| Body mass index (< 25 as reference) | ||
| 25 - 30 | 1.09 (0.88 - 1.35) | 0.42 |
| ≥ 30 | 1.06 (0.83 - 1.36) | 0.59 |
Multivariable Cox Regression Model for CHD
The HRs between study groups and CHD events were presented separately in males and females (Table 4). Compared to the reference group, the hazard ratios for all other study groups in both genders were both clinically important and statistically significant at 5% level, but with no important differences between genders (P for interaction = 0.65).
| Females (n = 3066) | Males (n = 2365) | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | P Value | HR (95 %CI) | P Value |
| Groups (History-Rose-ECG- as reference) | ||||
| History-Rose+ECG- | 2.06 (1.37 - 3.10) | < 0.001 | 2.37 (1.58 - 3.55) | < 0.001 |
| History-Rose-ECG+ | 2.27 (1.55 - 3.34) | < 0.001 | 1.66 (1.14 - 2.41) | 0.008 |
| History-Rose+ECG+ | 2.85 (1.32 - 6.13) | 0.007 | 2.23 (1.09 - 4.56) | 0.028 |
| CHD+ | 4.57 (3.27 - 6.40) | < 0.001 | 3.80 (2.84 - 5.08) | < 0.001 |
Multivariable Cox Regression Model for CHD Based on Gender Groups
Compared to the History-Rose+ECG- group, there were no significant differences in History-Rose-ECG+ (HR: 0.88; 95% CI: 0.62 - 1.26) and History-Rose+ECG+ (HR: 1.14, 95% CI: 0.64 - 2.02) groups. Considering History-Rose-ECG+ as the reference, no significant difference was detected in History-Rose+ECG+ group (HR: 1.29; 95% CI: 0.74 - 2.27).
In a separate analysis, all problematic participants with no history of CHD (including History-Rose+ECG-, History-Rose-ECG+ and History-Rose+ECG+) were compared with History+ patients. The significant differences were highlighted (HR: 3.34; 95% CI: 2.69 - 4.13).
With respect to the metabolic risk factors, the hazard ratios for History-Rose+ECG- and History-Rose-ECG+ were statistically as same as the HRs of hypertension and hypercholesterolemia.
Diabetes showed a statistically significant difference with History-Rose-ECG+ participants (HR of 3.04 vs. 1.92, P = 0.007) and borderline difference with History-Rose+ECG- group (HR of 3.04 vs. 2.18, P = 0.056).
5. Discussion
We compared the participants according to the presence of symptom (Rose Angina) or sign (ECG) or both/none of them at baseline. The risk of recurrent CHD among people with history of CHD was also evaluated. Our study with more follow-up time, confirmed the Khalili et al. findings that positive Rose Angina predicts the CHD events among Iranian people even in the presence of other cardiovascular risk factors, but not as much as diabetes (20).
Sensitivity and specificity of the Rose questionnaire may be different between countries (21). We found that Rose questionnaire as a good screening tool is appropriate to predict the risk and add information on undiagnosed CHD in both genders (HR = 2.37 in males and 2.06 in females). ECG has also been introduced as a strong predictive tool in some studies previously (22, 23).
The prediction power of ECG could not been found by Khalili et al. and the Rose-ECG+ groups had HR: 1.36 with a nonsignificant effect compared to baseline. Because no interaction was found between gender and Rose/ECG groups, we pooled both sexes for analysis. The analyses with more power showed the strong role of ECG to predict CHD in patients with silent ischemia.
However, we could define increased risk of CHD events among Rose+ECG+ population in parallel with the findings by Hemingway et al. (24). The limited sample size in this group might have caused the nonsignificant result through loss of power in the previous study.
We found no significant difference in the risk of CHD between Rose+ECG- and Rose-ECG+ in both genders. These findings revealed the same risk in symptomatic patients (Rose+ECG-) and the asymptomatic patients with positive sign (Rose-ECG+), especially in females.
More than 62% of males and 49% of females with self-reported history of CHD had experienced recurrent CHD during the follow- up. Considering participants without any history/symptom and sign of CHD as the reference, we found that the risk of new CHD in males and females with positive self-reported history were more than 3.8 and 4.57 folds, respectively.
In ARIC study, a population-based cohort of people aged 45 to 64 years, 766 CHD patients (189 females were followed for recurrent CVD events. During a mean of 8.7 years of follow-up, 313 acute CVD occurred, resulting in a recurrent CVD event rate of 47 per 1000 person-years (41 in females and 49 in males). The percentage of participants who had an acute CVD event by 10 years of follow-up was 38.7% for females and 45.1% for males (25).
The incidence of recurrent cardiovascular outcomes among patients with Type 2 diabetes was calculated by Giorda et al. They followed 2788 patients with diabetes aged 40 to 97 years with CVD at enrollment. During 4 years of follow-up, the incidence of a recurrent CVD was 72.7 per 1 000 person-years (95% CI: 58.3 - 87.1) and 32.5 per 1000 person-years (95% CI: 21.2 - 43.7) in males and females, respectively (26). Moreover, in another similar cohort study, with a median follow-up of 4.1 years, that was conducted by Heijden et al., the incidence rate of recurrent events per 100 person-years was 12.5 (8.5 - 17.6) in individuals with Type 2 diabetes (27). In Cha et al. study patients had recurrent episodes of CVD, with an incidence rate of 75.6 per 1000 patient-years (28).
Diabetic patients are more prone to have recurrent CVDs, and known diabetic patients demonstrated a CHD risk similar to nondiabetic patients with a prior CHD in both genders (29).
Increased risk of subsequent CVD morbidity and mortality is related to traditional risk factors, geographic location, and lack of treatment (30). Our study showed that hypertension and hypercholesterolemia had the same HRs as high as those for patients with positive findings in RQ or ECG. Moreover, it was found that diabetes has statistically and clinically important effects on CVD outcomes more than hypertension and hypercholesterolemia. For clinicians, prevention of new CVD and its recurrence in patients with previous CVD is an overwhelming task. We found that even participants with positive self-reported history had significant differences in either symptoms or signs compared to other problematic patients with negative history. Because the absolute risk is greater for this group, considering the high incidence rate of recurrent CHD in our population, this issue should seriously be considered in our country. Moreover, preventing first CHD in our high incident country is so important. The risk factors for recurrent CVDs are generally assumed tto be as same as the first ones, so controlling for the occurrence and proper interventions are necessary and results in preventing new and recurrent CVDs. There is ample evidence showing that a multiplicity of drug treatments and behavioral changes can reduce morbidity and mortality for those with existing CHD such as stopping smoking, maintaining a healthy diet, physical activity, and taking appropriate drug treatment (31).
Even with more analysis, we could not detect any statistically significant differences at 5% level for the risk of incident CHD between prevalent cases of CHD at baseline and those with no history of CHD who had both positive Rose Angina and abnormal ECG (HR: 1.66; 95% CI: 0.96 - 2.84; P = 0.06); and this might be a result of low power due to small sample size in history- Rose+ECG+ group. To our knowledge, no study was available which compared the risk of CHD in these 2 groups.
5.1. Study Strengths and Weaknesses
Our study had some restrictions, which should be kept in mind for better interpretation. First, these results cannot expand easily to the entire population, especially rural individuals. Secondly, both major and minor ECG abnormalities were considered as ECG abnormalities in our analysis. Because minor ECG abnormalities (ie, ST depression, T-wave items, small Q, or QS wave) may be related to other medical situations (ie, hyperventilation, anxiety, food ingestion, and change in posture), we might have attenuated the value of ECG changes in predicting incident CHD.
5.2. Conclusions
The rate of recurrent CHD in positive self-reported history of CHD in our community is high and it should be considered more precisely in practice. We found that each RQ and ECG has its own role in predicting CHD events. Rose questionnaire can be considered as a simple and helpful clinical screening tool among Iranian population with high prevalence of CHD even in the presence of normal ECG. However, ECG should be measured in the risk assessment of asymptomatic individuals. The predictive powers of these measurements were as same as that of hypercholesterolemia and hypertension, but lower than that of diabetes.