1. Context
Dyslipidaemia is a known risk factor for cardiovascular (CVD) and cerebrovascular occlusive events. Therefore, inhibition of cholesterol synthesis therapy plays a significant role in managing and preventing both of these diseases. Statin, a 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase inhibitor, is a class of drug used widely in dyslipidaemia. There are various types of statins prescribed worldwide. Atorvastatin (Lipitor and Torvast), simvastatin (Zocor and Lipex), lovastatin (Mevacor, Altocor and Altoprev), pitavastatin (Livalo and Pitava), rosuvastatin (Crestor), fluvastatin (Lescol), and pravastatin (Pravachol, Lipostat and Selektine) are the common types of statins found in the market. Each of these statins has a different effect on lipid profile. Lipid profile discussed in this review refers to the conventional lipid parameters that include total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C). Patients often have heterogeneous responses to different types of statins. Besides advanced age, gender, comorbidities, drug interaction, and interindividual variability, differences in pharmacokinetics, pharmacodynamics, and structure of each statin can lead to the diverse responses (1-4).
Asians have been less studied for the effects of statin on lipid profile compared to the Western population. Hence, it is necessary to study Asian population because there are large numbers of Asians throughout the world due to migration. Thus, the focus of this review was to discuss the effects of various types of established statins on lipid profile, particularly in Asians. Prior to discussing the effect of different statins on lipid profile, we will briefly discuss the structure of statin, its pharmacokinetics and mechanism of action, and its effects on lipid profile.
2. Evidence Acquisition
PubMed/MEDLINE searches were conducted using the keywords ‘statin, effect, and lipid profile’ from database inception through March 2016. In this review, 718 articles were retrieved from the primary search. After reviewing the titles, abstracts, and full texts of the studies, we found that 59 studies met our inclusion criteria. These also included subsequent reference searches of retrieved articles. All articles identified by this search were reviewed if the article text was written in English, related to effects of different types of statin (notwithstanding if the particular statin had been withdrawn from the market) on conventional lipid parameters such as total cholesterol (TC), triglycerides (TG), low density lipoprotein cholesterol (LDL-C), and high density lipoprotein cholesterol (HDL-C). Among the reviewed articles, 12 included statin prescription in Asian population.
3. Results
3.1. Structure of Statin
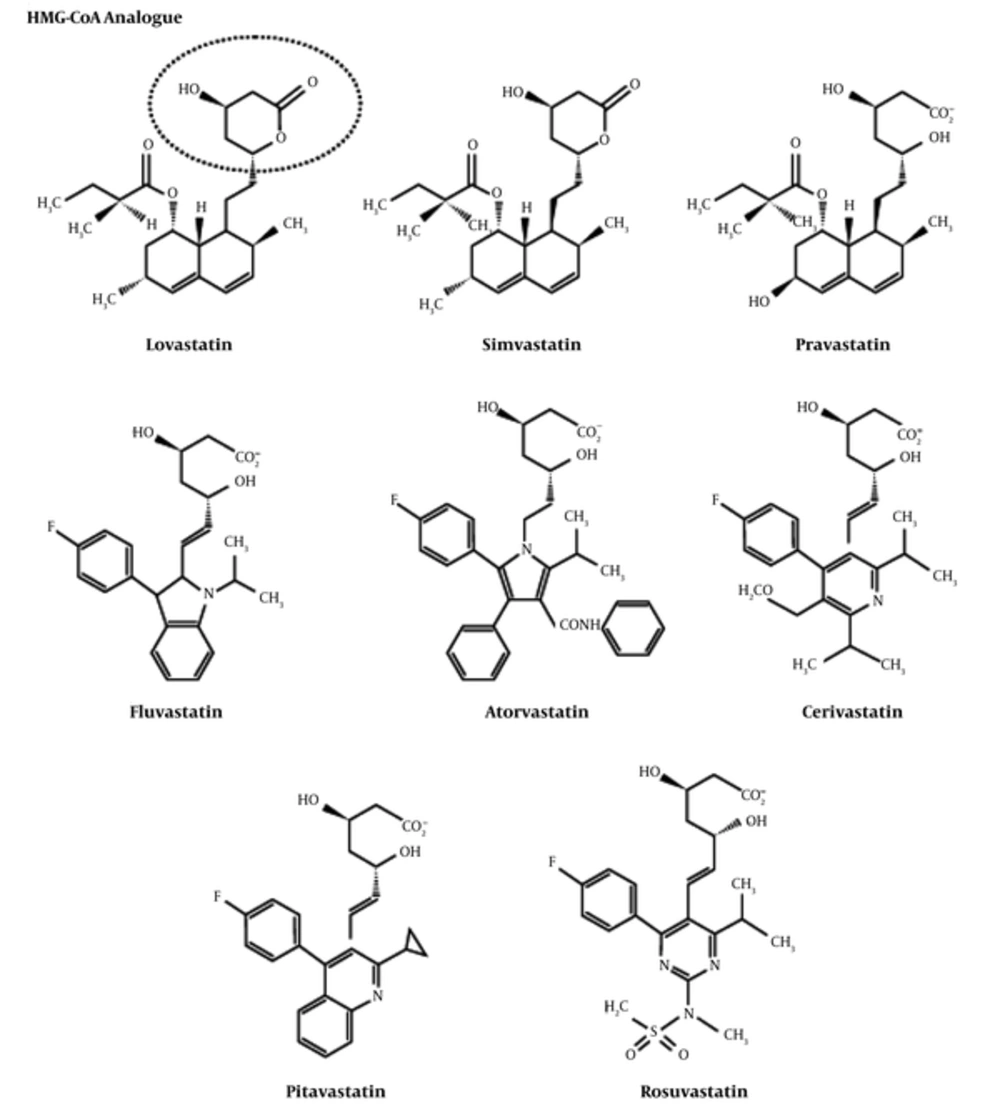
Statin can either be fungal-derived or synthetically produced. Lovastatin, pravastatin, and simvastatin are fungal-derived statins, while atorvastatin, cerivastatin, fluvastatin, pravastatin, pitavastatin, and rosuvastatin are fully synthetic compounds (5, 6). Generally, the chemical structures of statins, as shown in Figure 1, are divided into 2 segments (7):
i. The pharmacophore: Dihydroxyheptanoic acid segment, which is the analogue of the target HMG-CoA enzyme
ii. The pharmacophore moiety which is composed of the following:
• Complex hydrophobic ring structure that is covalently linked to the substrate analogue and involved in the binding with HMG-CoA reductase enzyme. The structure of the ring can be partially reduced naphthalene (lovastatin, simvastatin, pravastatin), a pyrrole (atorvastatin), an indole (fluvastatin), a pyrimidine (rosuvastatin), a pyridine (cerivastatin), or a quinoline (pitavastatin).
• Side groups on the rings define the solubility (hydrophobicity), and therefore, the pharmacokinetic properties.
Chemical Structures of Statins (5)
The functional difference between natural and synthetic statins relies on their ability to interact and inhibit the HMG-CoA reductase and on their hydrophobicity. Synthetically formed statins are known to form more interactions with HMG-CoA reductase because of their structural characteristics. Atorvastatin and rosuvastatin have additional hydrogen binding interactions. In addition, rosuvastatin also exhibits a polar interaction between the methane sulfonamide group and the HMG-CoA reductase enzyme. With these structural properties, rosuvastatin has the most efficient effect to reduce the activity of HMG-CoA reductase enzyme by 50% (6, 7). Pravastatin and rosuvastatin are more hydrophilic due to the polar hydroxyl group and methane sulphonamide group, respectively. Fluvastatin, lovastatin, atorvastatin, and simvastatin are relatively lipophilic compounds. The lipophilic statins (except pitavastatin and cerivastatin) have the properties of low systemic bioavailability because of an extensive first pass effect at the hepatic level (7, 8). The lipophilic statins can passively penetrate the cells of extrahepatic tissues, possibly leading to adverse events such as muscle toxicity. On the other hand, hydrophilic statins are more hepatoselective because they depend on an active transport mechanism to enter the liver; hence, they are excluded by extrahepatic tissues. However, the balance between desired and undesired effects of lipophilic and hydrophilic statins remains to be established (7).
3.2. Pharmacokinetics of Statins
Generally, statin enters the systemic circulation actively through the intestinal cells and passively via the ATP-binding cassette (ABC) and solute carrier family (SLC) gene family transporters. There are 2 families of enzymes responsible in statin metabolism, the cytochrome P450 (CYP), and UDP-glucoronosyltranferases (UGT), which mainly act in the liver, and to a lesser extent, in the kidney. Pharmacokinetics of each statin is based on their hydrophobic characteristic. For lipophilic statins (lovastatin, simvastatin, fluvastatin, and atorvastatin), transportation is via passive diffusion and is metabolised well by CYP enzymes. The main excretion pathway is through the biliary system. While the more hydrophilic statins such as rosuvastatin and pravastatin, active transport is the main mode of entry into the liver, they are metabolised less by the CYP enzymes and actively excreted through the kidneys. Lipophilic statins show an efficient activity at both hepatic and extrahepatic sites, whereas hydrophilic statins are more hepatoselective (7, 9).
Lipophilic statins have the general properties of low bioavailability because of first pass metabolism. Although fluvastatin, cerivastatin, and pitavastatin are lipophilic, their bioavailability are approximately 24% (19% - 29%), 60%, and 80%, respectively, which are significantly higher than rosuvastatin and pravastatin (7, 10-12). Pravastatin is the only statin not bound to plasma proteins. Hence, due to systemic exposure to unbound the drug, its level of pharmacological activity is relatively low, and the circulating level of this statin is higher compared to others (7, 13).
Pravastatin, cerivastatin, pitavastatin, rosuvastatin, and atorvastatin require organic anion transporting polypeptide C (OATP1B1) to cross the basolateral and apical membranes of hepatocytes (7, 14-16). Consumption of food has a variable effect on statin absorption (pharmacokinetic), which affects its bioavailability. The absorption of lovastatin is more effective when taken along with food, whereas the bioavailability of atorvastatin, fluvastatin, and pravastatin is decreased (5, 17-20). Food intake has no such effect on the bioavailability of simvastatin or rosuvastatin (1, 2, 9). Apparently, the time of consumption of statins does not affect the hypocholesterolemic effect (5, 17).
3.3. Mechanism of Action of Statins: Lipid Lowering Effect
Statin lowers plasma LDL-cholesterol (LDL-C) by competitively inhibiting the HMG-CoA reductase enzyme in the liver, the rate-limiting enzyme in cholesterol biosynthetic pathway. As a result, the conversion of HMG-CoA into mevalonate is inhibited, leading to the reduction of cholesterol produced. Hence, this reduction activates a transcription factor called sterol regulatory element binding protein (SREBP), which will be transported from the endoplasmic reticulum into the golgi apparatus. Transcription of LDL receptors will be activated, and thus, there will be an increase in the number of LDL receptors. Hence, the uptake of LDL-C will increase, reducing the plasma LDL-C level (21). Although statins share a common mechanism of action, there are differences in their relative efficacy for improving the lipid profile (5).
3.4. Effects of Different Types of Statin on Lipid Profile
According to the “Comparative Dose Efficacy Study of Atorvastatin Versus Simvastatin, Pravastatin, Lovastatin, and Fluvastatin in Patients with Hypercholesterolemia (the CURVES study, 1998)”, reduction of LDL-C was better with atorvastatin 10 mg compared to simvastatin 10 mg, pravastatin 10 mg and 20 mg, lovastatin 20 mg and 40 mg, and fluvastatin 20 mg and 40 mg (descending order of efficacy) (22). The same effect was found for TC, in which atorvastatin 10 mg, 20 mg and 40 mg had a better lowering effect compared to simvastatin, pravastatin, lovastatin, and fluvastatin (descending order of efficacy) at milligram-equivalent doses. However, for TG reduction, significant differences were found between atorvastatin and other statins at the 40 mg dose. The reduction of TG was greater with atorvastatin compared to simvastatin, fluvastatin, pravastatin, and lovastatin (descending order of efficacy). Similarly, when elevating HDL-C, significant results were only observed at 40 mg dose. However, this effect was greater with simvastatin compared to atorvastatin. The CURVES study was comparable to previous research on lipid-lowering effects of statins (22, 23). The reported reductions in LDL-C were 28% - 41%, 18% - 34%, 25% - 38%, and 18% - 27% for simvastatin (10 mg - 40 mg), pravastatin (10 mg - 40 mg), lovastatin (20 mg - 40 mg), and fluvastatin (20 mg - 40 mg), respectively. While for atorvastatin, the reduction in LDL-C was 35% to 61%, identifying atorvastatin as the most efficient statin (22). This conclusion was consistent with prior comparative studies that involved atorvastatin (24-26).
In statin therapies for elevated lipid levels compared across doses to rosuvastatin (STELLAR trial), Jones et al. established results based on the dose-to-dose comparisons between atorvastatin and rosuvastatin efficacy in reducing LDL-C (27). The results revealed that the efficacy of rosuvastatin 40 mg had the best efficacy with 55.0% LDL-C reduction (-55%), followed by rosuvastatin 20 mg (-52.4%), atorvastatin 80 mg, atorvastatin 40 mg (-47.8%), rosuvastatin 10 mg (-45.8%), atorvastatin 20 mg (-42.6%), and atorvastatin 10 mg (-36.8%). The reduction of LDL-C by rosuvastatin 10 mg, 20 mg and 40 mg were significantly greater than simvastatin and pravastatin in all 14 pairwise comparisons. The rank order of effects of prescribed statins related to the LDL-C reduction for all 14 pairwise comparisons is shown in Table 1. Moreover, their study also showed the association of types of statins with the elevations of HDL-C. As the doses of rosuvastatin, simvastatin, and pravastatin increased, the elevation of HDL-C also increased. HDL-C increment was significantly greater for rosuvastatin compared to simvastatin and pravastatin. In contrast, the elevation of HDL-C by atorvastatin decreased across the dose range. Rosuvastatin 40 mg had the greatest increasing effect on HDL-C, while atorvastatin 80 mg had the least (+ 9.6% vs. + 2.1%). Across the dose range, rosuvastatin 10 mg - 80 mg reduced the TC 4.7%, 9.0%, 12.5%, and 18.7% more than atorvastatin 10 mg - 80 mg, simvastatin 80 mg, simvastatin 10 mg, and pravastatin 10 mg - 40 mg, respectively. For the reduction of TG, rosuvastatin 10 mg – 80 mg had a similar effect to atorvastatin 10 mg - 80 mg, but a better effect compared to simvastatin 10 mg - 80 mg (+ 7.5%), and pravastatin 10 mg - 40 mg (+ 18.1%). This study also stated that rosuvastatin 20 mg - 40 mg groups had the highest percentage of patients who met the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) and European LDL-C goals [100 mg/dL (2.6 mmol/L) and 116 mg/dL (3.0 mmol/L), respectively] (28, 29). The percentage of patients from rosuvastatin 10 mg group who reached both of these goals was similar to the percentage reached by the groups prescribed with the highest doses of atorvastatin and simvastatin (80 mg) (27).
| Statins and Doses | STELLAR Trial (27) | Comparative Trials (5) | |||
|---|---|---|---|---|---|
| LDL-Ca Reduction (20 - 55)% | LDL-Ca Reduction (24 - 63)% | HDL-C Increase (6 - 12)% | TG Reduction (10 - 29)% | ||
| 1 | Rosuvastatin 40 mg | 55.0 | 63 | 10 | 28 |
| 2 | Rosuvastatin 20 mg | 52.4 | |||
| 3 | Atorvastatin 80 mg | 51.1 | |||
| 4 | Atorvastatin 40 mg | 47.8 | 50 | 6 | 29 |
| 5 | Rosuvastatin 10 mg / Simvastatin 80 mg | 45.8 | |||
| 6 | Atorvastatin 20 mg | 42.6 | |||
| 7 | Pitavastatin 4 mg | 48 | no significant effect | 23 | |
| 8 | Simvastatin 40 mg | 38.8 | 41 | 12 | 18 |
| 9 | Atorvastatin 10 mg | 36.8 | |||
| 10 | Simvastatin 20 mg | 35.0 | |||
| 11 | Pravastatin 40 mg | 29.7 | 34 | 12 | 24 |
| 12 | Lovastatin 40 mg | 34 | 9 | 16 | |
| 13 | Pravastatin 20 mg | 24.4 | |||
| 14 | Pravastatin 10 mg | 20.1 | |||
| 15 | Cerivastatin 0.3 mgb | 28 | 10 | 13 | |
| 16 | Fluvastatin 40 mg | 24 | 8 | 10 | |
The Effects of Different Statins on Lipid Profile (27)
Similarly, data from comparative trials, shown in Table 1 confirm that rosuvastatin is the most effective statin for lowering LDL-C (reduction of 63% with 40 mg daily), followed by atorvastatin, simvastatin, and pravastatin. The lipid lowering effect of pitavastatin was comparable to atorvastatin. Statins also increased HDL-C levels to varying degrees, although not a predictable dose-response relationship (5).
A study by McTaggart et al. comparing the potency of atorvastatin, cerivastatin, fluvastatin, pravastatin, rosuvastatin, and simvastatin identified rosuvastatin as the most potent statin (30). The results showed that the 50% inhibitory concentration (IC50) of rosuvastatin was 0.16 nM, followed by atorvastatin (1.16 nM), and pravastatin was the least potent statin with IC50 of 6.93 nM. The terminal half-life of rosuvastatin was approximately 20 hours, which was similar to atorvastatin (5, 30). Kapur et al. suggested that the high potency of rosuvastatin was due to its unique chemical characteristics (31). First, the fluorinated phenyl group and a polar methane sulphonamide group of rosuvastatin provide multiple sites of activity against HMG-CoA reductase (32). Second, rosuvastatin has enhanced binding enthalpies, which is defined as the strength of the interaction between the inhibitor and target enzyme for HMG-CoA reductase. Rosuvastatin and atorvastatin are the only statins to form a hydrogen bond with the hydroxyl group of Ser565 on HMG-CoA reductase enzyme. A shorter hydrogen bond length and involvement of sulfonyl groups contribute to superior enzyme inhibition by rosuvastatin (33). Third, the hydrophilic nature of rosuvastatin may also help eliminate the dependence on metabolic conversion to a water-soluble molecule, especially in the presence of other drugs (34).
In a study comparing the effect of pitavastatin with pravastatin, Saito et al. stated that the effects of pitavastatin was greater than pravastatin on the reduction of LDL-C (- 37.6% vs. - 18.4%) and TC (- 28.2% vs - 14.0%) (35). Pitavastatin also reduced the levels of TG, apolipoprotein (apo) B, C-II, and CIII at a greater extent compared to pravastatin. However, elevations of HDL-C, apo A-I and A-II were similar between the 2 statins. After 12 weeks, 75% of the subjects prescribed with pitavastatin met the LDL-C target level of < 140 mg/dL (3.6 mmol/L) compared to 36% from the pravastatin group (35).
Yokote et al. conducted a 12-week, prospective, open-label trial to compare the efficacy and safety of pitavastatin 2 mg/day against atorvastatin 10 mg/day (36). There were no significant differences between pitavastatin 2 mg and atorvastatin 10 mg effects on the reduction of LDL-C, TC, and TG. However, the elevations of HDL-C levels were much more significant in pitavastatin 2 mg compared to atorvastatin 10 mg. The waist circumference, body weight, and body mass index (BMI) were significantly correlated with the percentage of serum non-HDL-C reduction in the atorvastatin group, whereas consistent reduction of serum non-HDL-C was observed in the pitavastatin group, regardless of body size (36). Data on pitavastatin relative potency are limited, however, Kajinami et al. stated that pitavastatin was 2.4-fold and 6.8-fold more potent than simvastatin and pravastatin, respectively, with an IC50 of 6.8 nM (10).
A randomised trial comparing the safety, tolerability, and efficacy of simvastatin 20 mg with pravastatin 20 mg in 210 patients with primary hypercholesterolemia, found that simvastatin had a significantly better effect on lipid profile compared to pravastatin. The results compared the effects of simvastatin and pravastatin on the mean reductions from baseline levels of TC (28% versus 21%), LDL-C (38% versus 29%), and apo B (25% versus 17%), respectively. A reduction of 20% in LDL-C was identified in 94% of patients receiving simvastatin 20 mg and 80% of patients receiving pravastatin 20 mg. There were, however, similar significant reductions from baseline in TG (14% and 11%), elevation in HDL-C (7% for both), and apolipoprotein A-I (4% for both) for both simvastatin and pravastatin, respectively (37).
A similar study by the Simvastatin Pravastatin Study Group (2013) also compared the effects of simvastatin and pravastatin, with 30% of simvastatin group and 14% of pravastatin group on 10 mg dose, while, 48% of simvastatin group and 66% of pravastatin group were titrated to the maximal dose (38). After 18 weeks, the mean reduction for TC was 27% and 19%, it was 38% and 26% for LDL-C, 30% and 16% for very low-density lipoprotein cholesterol (VLDL-C), and 18% and 14% for TG for simvastatin and pravastatin, respectively. Simvastatin also produced better results for HDL-C elevation compared to pravastatin (+ 15% vs. + 12%). The efficacy goal of LDL-C < 130 mg/dL (3.4 mmol/L) was achieved in 65% of the patients treated with simvastatin versus 39% of those treated with pravastatin (38).
Berger et al. stated that the effect of lovastatin on lipid profile (TC, TG, LDL-C, and HDL-C) was significantly more potent than fluvastatin on a per milligram basis (39). After 6 weeks, the results showed that reductions in TC and LDL-C with lovastatin 20 mg were - 19.5% and - 27.6%, respectively, while the reductions were 12.8% and 18.2%, respectively, with fluvastatin 20 mg.
3.5. Use of Statins in Asians
Similar to Western countries, statins are also widely prescribed across Asia as a primary and secondary prevention of CVD and ischaemic stroke (40-42). Although the incidence of coronary heart disease (CHD) is lower in Asian populations compared to Caucasians, Asians have a higher incidence of stroke (43, 44). Stroke is the leading cause of death in China and the third leading cause of mortality in Malaysia (44, 45). A study in Singapore confirmed that the prevalence of stroke incidence among the main races (Malays, Chinese, and Indians) is similar (44, 46).
Studies have demonstrated that Asians require a lower dose of statins compared to Caucasians to achieve comparable target lipid profiles. The heightened response to statins in Asians could be attributed to the genetically based differences in the metabolism of statins at the level of hepatic enzymes and drug transporters (47). Due to this increased systemic exposure to statins, it is advisable to commence treatment with lower dose in Asians to maintain safety and efficacy of the drug (47).
However, the study by Gandelman et al. revealed contradictory results. In a combined analysis and comparison of 22 pharmacokinetic studies of atorvastatin, it was concluded that there were no differences in the systemic exposure to atorvastatin between Asian and Caucasian participants (48). The dose-per-bodyweight normalised area under the concentration-time curve values (AUCdn,wt) for atorvastatin was 157.5 (ng.hr.mL-1)/(mg.kg-1) for Asians and 156.4 (ng.hr.mL-1)/(mg.kg-1) for Caucasians, while the dose-per-bodyweight normalised maximum observed concentration (Cmaxdn,wt) for Asians was 26.2 (ng.hr.mL-1)/(mg.kg-1) and 30.3 (ng.mL-1)/(mg.kg-1) for Caucasians. Therefore, the dosing considerations for atorvastatin were similar for both Asians and Caucasians (48).
Gupta et al. evaluated the lipid-modifying effects of statins in South Asian and Caucasian patients with established CHD (49). The results showed that atorvastatin 20 mg produced similar reductions in LDL-C in both South Asian (-43%) and Caucasian (-41%) patients. The elevations of HDL-C were + 19% in South Asians and + 12% in Caucasians. Simvastatin 20 mg reduced LDL-C by - 35% in South Asians and by - 37% in Caucasians, while elevating HDL-C levels by + 12% in both groups. Using a multiple linear regression model, this study suggested that the expected reduction in LDL-C for atorvastatin 10 mg and atorvastatin 20 mg was similar between the groups. The results showed that atorvastatin and simvastatin, at commonly prescribed doses, modulated LDL-C and HDL-C levels to a similar degree in both South Asians and Caucasians with documented CHD. Hence, South Asian patients may be treated with similar doses of atorvastatin and simvastatin as prescribed to Caucasians (49).
A study by Deedwania et al., which compared the effect of rosuvastatin and atorvastatin in South Asian patients who were at risk of CHD, identified that the LDL-C decreased better in rosuvastatin 10 mg group compared to atorvastatin 10 mg group (- 45% vs. - 40%), and in rosuvastatin 20 mg group compared to atorvastatin 20 mg group (- 50% vs. 47%) (58). NCEP ATP-III LDL-C goal achievement rates in high-risk patients (all patients) were 76% (79%) and 88% (89%) with rosuvastatin 10 mg and 20 mg, respectively, compared with 70% (76%) and 81% (85%) with atorvastatin 10 mg and 20 mg, respectively. Both statins were well tolerated with no clinically relevant differences in adverse events or incidence of creatine kinase > 10 times higher than the normal upper limit. Hence, it was concluded that rosuvastatin 10 mg and 20 mg and atorvastatin 10 mg and 20 mg were effective in achieving the target LDL-C and were well tolerated in South Asian population (50).
A study (The Direct Statin Comparison of LDL-C Values: An Evaluation of Rosuvastatin Therapy (DISCOVERY, 2007)- Asia) was conducted to evaluate the lipid-lowering effects of rosuvastatin and atorvastatin in Asian patients (51). The main objective of this study was to assess the efficacy of initial doses of statins in achieving target lipid levels in 6 different Asian countries (China, Hong Kong, Korea, Malaysia, Taiwan and Thailand). Rosuvastatin 10 mg/day or atorvastatin 10 mg/day were randomly prescribed in a 2:1 ratio to 1482 adults with primary hypercholesterolemia and high cardiovascular risk (> 20%/10 years, Type 2 diabetes, or a history of CHD). The results showed that the percentage of patients achieving the 1998 and 2003 European goals for LDL-C and TC at 12 weeks were higher in rosuvastatin group compared to atorvastatin group. This study concluded that to achieve the European goals for LDL-C and TC in Asian clinical practice, the starting dose of rosuvastatin 10 mg/day was significantly more effective than the starting dose of atorvastatin 10 mg/day. The safety profile of both rosuvastatin 10 mg and atorvastatin 10 mg was similar in Asians and was consistent with the established safety profile of rosuvastatin in the Caucasian population. Nonetheless, the United States Food and Drug Administration (FDA) recently advised the initiation of rosuvastatin at 5 mg/day in Asian patients (47).
4. Conclusions
In summary, there are significant differences in the relative effects of different types of statins on the improvement of lipid profile. Rosuvastatin is considered as the most potent and efficient statin in managing dyslipidaemia. Although atorvastatin has a significant effect on non-HDL-C profile, its effect on HDL-C is the lowest compared to all other statins. To balance the benefits and risks of statins in patients, 2 facts should be emphasised: statins that have great benefits in decreasing cardiovascular and cerebrovascular events, and those best tolerated statins need to be identified. There is still lack of firm evidence on the association on the types of statins, which are responsible for statin efficacy and statin intolerance in Asians. Thus, further large prospective trials should be conducted to establish concrete associations.