1. Background
Emerging evidence suggests that enhanced arginase activity and expression is involved in the conditions characterized by vascular endothelial dysfunction including hypertension, atherosclerosis, diabetes, and aging both in experimental animal models (1, 2) and patients with diabetes (2-4). Nitric oxide synthase (NOS), in the vascular endothelial cells, consumes L-arginine (L-Arg) to produce NO, which regulates blood flow and decreases inflammation (5, 6). Decreased availability of L-Arg to NOS and subsequently reduction of NO production have been contributed to the vascular dysfunction associated with diabetes. Deficiency in the L-Arg availability for NOS can occur by over expression or increased activity of arginase, an enzyme that competes with NOS for the use of common substrate, L-Arg (7). Arginase (L-Arginine urea hydrolase, EC 3.5.3.1), which catalyzes the hydrolysis of L-arginine to urea and L-ornithine, exists in two isoforms, the hepatic type, arginase I, and the extra-hepatic type, arginase II, which are found within cytosol and mitochondria, respectively (7-9) and are found in endothelial cell populations (4, 7). It has been reported arginase I activity is enhanced in diabetic retinopathy, asthma and sickle cell disease, while arginase II has been implicated specifically in retinopathy of premature atherosclerosis and diabetic renal injury (10). Studies have shown that vascular dysfunction in diabetes is directly correlated with oxidative stress and inflammation (11, 12), both of which have been correlated with upregulation of arginase activity and expression (13, 14). In fact, inflammatory molecules, which are increased in hyperglycaemia including cytokines, oxidative radicals and glucose, activate the arginase pathway, whereas, simultaneously, they can reduce the eNOS pathway (7, 15, 16). Therefore, L-Arg pool insufficiency can cause the NOS homodimer to become uncoupled, leading to produce superoxide. Superoxide and any available NO can interact to form a strong biological oxidant, peroxy-nitrite anion (ONOO-), further inducing excessive oxidative stress and less NO production (14, 17). Peroxynitrite can mediate cellular damage via direct oxidative interactions with lipids, DNA and proteins or via indirect (17). On the other hand, an excessive activity of the arginase pathway participates in promoting abnormal vascular growth and stiffness through increasing production of polyamines and proline (18). This offers that arginase can be a therapeutic target for diabetic retinopathy.
Zingiber Officinal Roscoe (Z. officinale), a widely-utilized spice worldwide, has been commonly considered as a traditional herbal medicine, exhibiting antioxidant, anti-inflammatory, and antidiabetic properties (19-21). It also possesses protective effects on diabetic complications (19). In addition, some in vivo and in vitro studies have reported that Z. officinale can delay the development of diabetic cataract (22-24). According to the aforementioned, we hypothesized that Z. officinale might diminish arginase activity. This study aimed at investigating the effect of the Z. officinale methanolic extract on the arginase activity and its protein level in vivo using the streptozotocin (STZ)-induced diabetic rat model.
2. Objectives
The current study was designed to determine the effects of the hydroalcoholic extract of Z. officinale on the arginase I activity and expression in the retina of STZ-induced diabetic rats.
3. Methods
3.1. Animals
A total 16 male Wistar rats with an average weight of 200 - 250 g (8 - 10 weeks of age) were studied. The rats were purchased from the research center and experimental animal house of Ahvaz Jundishapur University of Medical Sciences (Ahvaz, Iran). The study was approved in accordance with the ethical committee of animal breeding and research of Jundishapur University of Medical Sciences (approval number: MPRC-9406, approval date: 2015/7/1). Upon arrival, the animals were housed in a well-ventilated room with a relative temperature of 22 ± 2°C and photoperiod (12-hour light/dark cycle). Animals were given free access to standard food pellet and tap water during the experiment.
3.2. Experimental Design
After one-week adaptation, following an overnight fasting, type 1 diabetes mellitus was induced by a single intraperitoneal injection of STZ (Sigma-Aldrich, USA, 60 mg/kg, freshly dissolved in 0.1 M cold citrate buffer, pH 4.5) (25). The healthy control group was injected with the same volume of the sodium citrate buffer solution alone. Three days after STZ administration, the rats, which have nicked tail-vein blood glucose concentration more than 350 mg/dL were considered diabetic. Oral gavage of the Z. officinale extract was initiated two weeks after the STZ injection and continued for a period of 8 weeks. The rats were randomly categorized into four experimental groups of four rats in each group as follows:
Group 1: The untreated healthy control group was gavaged with 1.5 mL/kg distilled water once daily for 8 weeks. Group 2: The untreated diabetic control group gavaged with 1.5 mL/kg distilled water once daily for 8 weeks. Group 3: Diabetic rats gavaged orally with the Z. officinale extract at a dose of 200 mg/kg body weight in distilled water (1.5 mL/kg) once daily for 8 weeks. Group 4: Diabetic rats gavaged orally with the Z. officinale extract at a dose of 400 mg/kg body weight in distilled water (1.5 mL/kg) once daily for 8 weeks.
3.3. Sample Collection
At the end of the eight-week treatment, the fasted rats in all groups were weighed and then sacrificed after anesthetizing. Fresh blood samples were directly obtained via cardiac puncture using not heparinized syringes. Subsequently the eye balls were immediately collected and washed in cold normal saline solution, then snap-frozen in liquid nitrogen and stored at -80°C for later assay.
3.4. Biochemical Analysis
Sera were separated by centrifugation of whole blood at 3000 g for 15 minutes at 4°C and used for measurement of serum glucose and insulin concentration. Fasting blood glucose concentration was determined using the commercial available colorimetric kit (Pars Azmoon co, Tehran, Iran) with an automatic biochemical analyzer. Serum insulin concentration was determined using a commercial available rat insulin ELISA kit (Thermo Scientific Inc., Rockford, IL).
3.5. Preparation of the Zingiber officinale Methanolic Extract
Dried roots of Z. officinale (Z. officinale, family Zingiberaceae) were obtained from Gol Darou Company, Isfahan, Iran. The hydroalcoholic extract of Z. officinale was made through the maceration method. For extraction, dried Z. officinale roots (200 g) were powdered using an electric blender, soaked in 1400 mL 0f 70% methanol solution (v/v), and then kept at the room temperature for 72 hours. The infusion was then filtered through the Wattman filter no.4 and the solvent was evaporated using a rotary evaporator. To increase the shelf life and homogeneity, the extract was lyophilized completely by a continuous freeze-drying operation and the yield was 12.5% (w/w), which was stored at -20°C until use (26).
3.6. Assay of Arginase Activity
The assay was conducted by spectrophotometric measurement of urea production to confirm the efficacy of the Z. officinale effect on the arginase activity in the retina lysates. Briefly, first the whole eyes of rats were gently harvested and the retinas were peeled away. Then retina were sonicated in ice-cold lysis buffer containing 50 mM Tris-HCl (pH 7.5), 0.1 mM EDTA, 0.1 mM EGTA, 0.1% TritonX-100 and freshly dissolved protease inhibitor. The samples were subsequently lysed by repeated freezing and thawing (3 cycles) and the lysates were then centrifuged at 16000 g at 4°C for 20 min to remove insoluble materials. Afterwards, 25 µL MnCl2 (10 mM in 50 Mm Tris-HCl) was added to 25 µL supernatant and heated at 56°C for 10 min to activate the enzyme. Next, to initiate enzyme activity, assay solution was added to 50 µL L-Arg (0.5 M in 50 mM Tris-HCl, pH 9.7) and then incubated at 37°C for 1 hour. The arginase reaction was stopped by adding 400µL of an acid mixture (H2SO4:H3PO4:H2O in a 1:3:7 ratio). For colorimetrical determination of urea, the mixture reaction was heated with 25 µL of chromogenic α-isonitrosopropiophenone (9% in ethanol) at 100°C for 45 minutes and then placed in the dark at room temperature for 10 minutes. Finally, the absorbance was read by a spectrophotometer at a wavelength of 540 nm. Arginase activity was expressed as picomoles of urea production per milligram of protein per hour. The blank solution contains all of the above-mentioned compounds except MnCl2 or L-Arg.
3.7. Western Blot Analysis
Western blot analysis was performed on the supernatant fraction of homogenized rat retinas. Total protein concentrations of the supernatant were determined by the Bradford method. Fifty micrograms (50 μg) protein from each sample was loaded onto 10% odium dodecyl sulfate (SDS)-polyacrylamide gels for electrophoresis (SDS-PAGE). Protein blots were then electro- transferred to a methanol-preactivated polyvinylidene difluoride (PVDF) membrane in Tris-glycine buffer by BIO-RAD transfer system (USA). The membrane was blocked with bovine serum albumin (BSA) 5% in Tris-buffered saline plus 0.1% Tween-20 (TBST) for 1 hour with gently shakes. Subsequently, blots were probed with antibodies specific for Arg-I (1:200 dilution) (sc-20150; Santa Cruz Biotechnology), and then reprobed for β-actin (sc-130656; Santa Cruz Biotechnology) to ensure equal protein loading. Membrane bound antibody was incubated with secondary antibody, goat anti-rabbit IgG-HRP (sc-2030; Santa Cruz) diluted 1:10000 in 3% BSA for 1 hour at the room temperature. After each step, membranes were washed thrice with TBST. Finally, the blots were visualized with the Chemi-Doc gel documentation system (Bio-Rad, Hercules, CA) using an enhanced chemiluminescence (ECL) western blotting detection kit according to the manufacturer’s protocol. Optical densities of bands were measured using Image J software and quantified as the ratio to β-actin. The mean value of samples for the healthy control rats in each blotting, expressed as the densitometry unit was adjusted to a value of 1.0. All experimental sample values were expressed relative to this adjusted mean value.
3.8. Statistical Analysis
All statistical analyses were performed with the SPSS software (SPSS Inc, Chicago, Illinois, USA). Results were reported as means ± standard deviation. A value of P < 0.05 was regarded as statistically significant. One-way ANOVA followed by Tukey’s post-hoc test was used for statistical comparisons of data with normal distribution (including blood glucose and body weight) and data with no normal distribution (including insulin, arginase activity, and expression) were analyzed by the Kruskal-Wallis test. Illustrations were done using the GraphPad Prism software (GraphPad Software Inc, San Diego, CA, USA).
4. Results
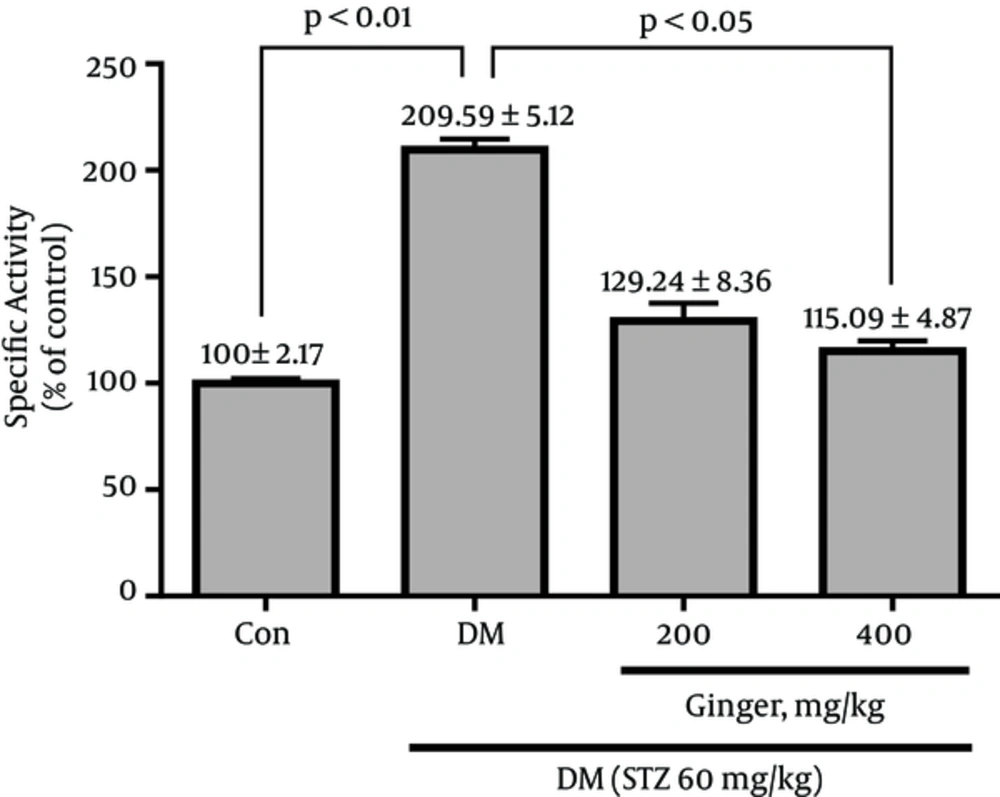
All rats in the four studied groups remained active throughout the experimental period. Before diabetes induction, there was no statistically significant difference in the body weight and fasting blood glucose concentration among four groups. The effects of the Z. officinale extract on body weight, fasting serum insulin and glucose, arginase activity, and arginase I protein level was ascertained by comparison of diabetic controls with healthy controls and treated diabetic with untreated diabetic controls.
4.1. Effects of Zingiber officinale Extract on the Body Weight
As shown in Table 1, at the end of the study, STZ-induced diabetes resulted in a significant reduction of body weight in the untreated diabetic controls compared to the healthy controls (P < 0.01). However, body weight was significantly elevated in both treated diabetic groups compared to the untreated diabetic controls and this effect was more obvious in diabetic rats which received 400 mg/kg of the extract than those received 200 mg/kg of the extract (P < 0.01 and P < 0.05, respectively).
Abbreviation: DM, diabetes mellitus.
aValues are expressed as mean ± SD (n = 4).
bP < 0.01 vs. healthy control.
cP < 0.05 vs. diabetic control.
dP < 0.01 vs. diabetic control.
4.2. Effects of the Zingiber officinale Extract on the Biochemical Parameters
As expected and shown in Table 1, fasting serum insulin was significantly decreased in the STZ-induced diabetic rats compared to the healthy controls (P < 0.01). Conversely, treatment with 400 mg/kg of the Z. officinale extract for eight weeks was significantly increased fasting serum insulin compared to the diabetic controls (P < 0.05). However, no statistically significant difference was observed in the fasting serum insulin concentration between diabetic treated with 200 mg/kg of the Z. officinale extract and diabetic controls (P = 0.08).
As shown in Table 1, the untreated diabetic rats showed a markedly higher fasting blood glucose concentration than the healthy control group (P < 0.01). While, after an eight-week treatment, blood glucose was significantly decreased in both Z. officinale treated diabetic groups compared to the untreated diabetic rats (P < 0.01). In addition, consumption of Z. officinale at a daily dose of 400 mg/kg led to a greater effect in decreasing the fasting blood glucose concentration, which was not statistically different when compared to healthy controls (P = 0.17). This indicates that 400 mg/kg Z. officinale treatment for 8 weeks normalized the fasting blood glucose concentration. Results are represented in Table 1.
4.3. Effects of the Zingiber officinale Extract on the Activity and Protein Level of Arginase I
As illustrated in Figure 1, at the end of the experimental period, a significant increase was observed in the arginase activity (109.6%) in the untreated diabetic rats compared to the healthy controls (P < 0.01). However, treatment with 400 mg/kg of the extract resulted in a remarkable decrease in arginase activity compared to the untreated diabetic controls (P < 0.05). While, no statistically significant difference in the arginase activity was observed between diabetic rats treated with 200 mg/kg of the extract and untreated diabetic controls (P = 0.21). Results are reported as percentage of control.
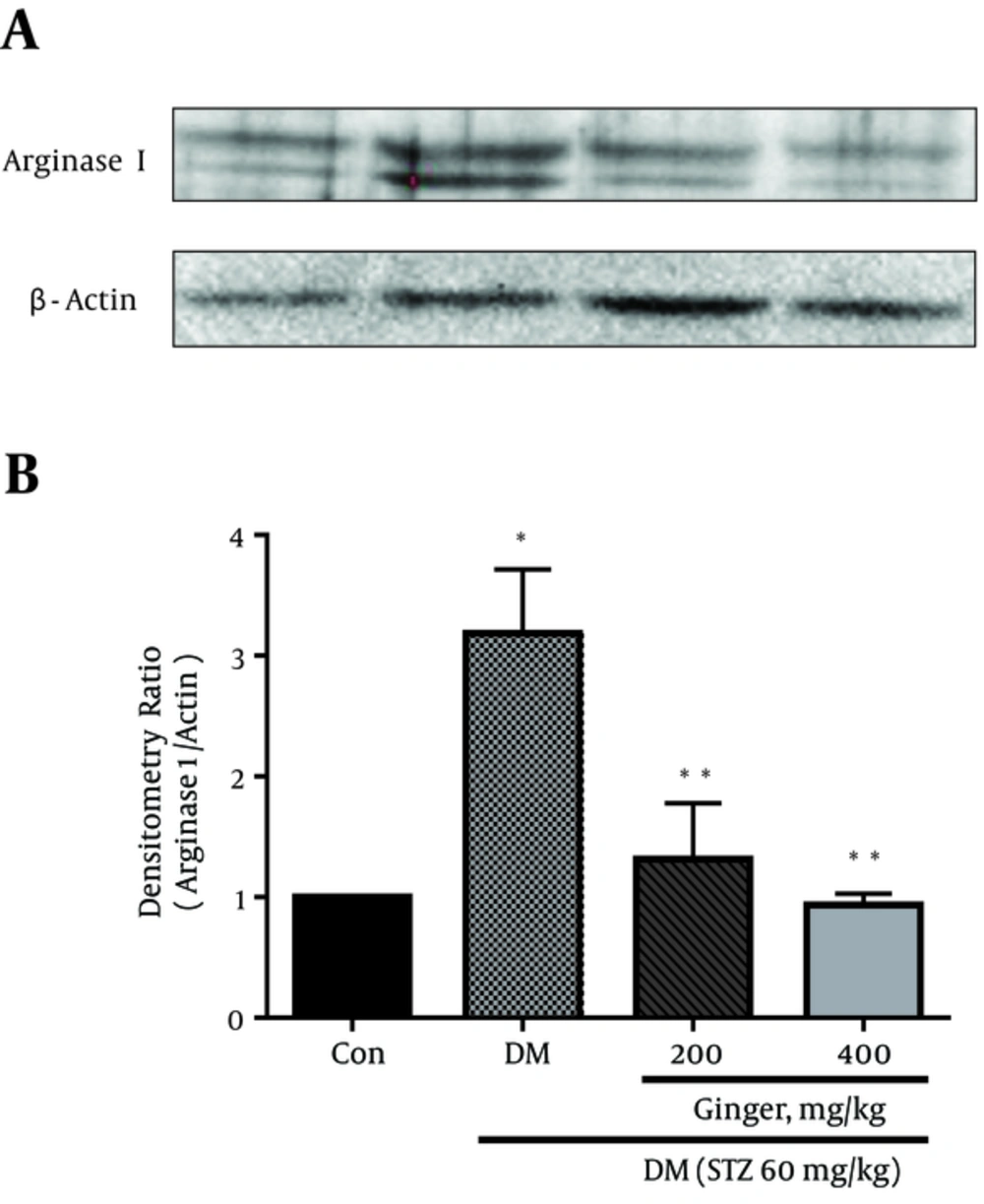
Similarly, the western blot analysis exhibited that the arginase I protein level was significantly increased (3.2 fold) in the retina of the STZ-induced diabetic rats compared to the healthy controls (P < 0.01). Whereas, a significant reduction was observed in the arginase I protein level in diabetic rats treated with 200 and 400 mg/kg of Z. officinale extract as compared to the untreated diabetic controls, more effect was observed in rats treated with 400 mg/kg of the extract (P < 0.01) (Figures 2A and 2B).
A, Representative immune-blots demonstrating specific bands for arginase I; B, Graphical presentation of data obtained from four independent experiments from the western blot analysis. The mean value of arginase I is expressed as the ratio of arginase I to β-actin in each column (n = 4). β-actin was applied as an internal control. Error bars show S.D. *P < 0.05 vs. healthy control; and **P < 0.05 vs. diabetic controls.
5. Discussion
Despite major progress for hyperglycemia control through diet therapy, pharmacological agents, insulin and islet transplantation, management of long-term diabetic complications such as blindness still remains a major clinical challenge (23). Arginase, a key enzyme of urea cycle, mainly involved both in vascular and neuronal damage and the arginase/polyamine pathway plays an important role in the diabetic neurovascular damage in the retina (17).
Since some conventional medications marketed for diabetic complications have troublesome side-effects, interest in natural complementary and alternative therapies is increasing. In traditional medicine, plants have been considered as a rich source of therapeutic compounds for many indications. A number of these herbal medicines may be promising as therapies for diabetes and hyperglycemia, an important one is Z. officinale the blood glucose lowering effect of which has been observed and supported by recent in vivo and in vitro studies (19-24). Since no study regarding the effects of Z. officinale hydroalcoholic extract on the arginase expression and activity in diabetes has ever been performed, the current study aimed at examining the effects of Z. officinale hydroalcoholic extract, specifically in an animal model.
Our results indicated that body weight significantly decreased in the untreated diabetic rats compared to the healthy controls. In a study conducted by Kusari et al. loss of body weight in the STZ-induced diabetic rats had been attributed either to the enhancement of urine output, which leads to dehydration and loss of valuable fluids, or to muscle breakdown resulting from hyperglycemia (27). Our results are also in agreement with the results of Eleazu et al. who reported the cause of weight loss in the STZ-induced-diabetic rats resulted from the loss and degradation of structural proteins (28). We found that the Z. officinale extract protects the STZ-induced diabetic rats against weight loss and this effect may be due to its potential hypoglycemic properties. Moreover, this result is in agreement with the findings reported by Faried et al. and Thomson et al. (23, 29).
In the current study, following 10 weeks of diabetes induction, serum insulin significantly decreased in the untreated diabetic controls compared to healthy controls. In line with these findings, Zafar et al. observed that the diabetogenic effect of STZ is a direct result of the irreversible damages of β cells, which leads to degranulation and decrease of insulin secretion (30). In addition, our results showed that serum insulin in diabetic rats treated with 400 mg/kg of the Z. officinale extract significantly increased relative to the untreated diabetic group, results consistent with those reported by Iranloye et al., and Akhani et al. (31, 32); according to their results, they speculated that the Z. officinale extract may be attributed to increased insulin secretion from β cells or release of granule-bounded insulin (31). Heimes et al. suggested that the effects of Z. officinale in the retrieval of insulin may be due to its reaction with the 5-Hydroxy-tripetamin (5-HT3) receptor (33). Several studies have confirmed that serotonin receptors may be involved in the hypoglycemic effects of the Z. officinale extract. Serotonin receptors suppress the release of insulin and the Z. officinale extract can antagonize this inhibitory effect (34).
We found that, accompanied by a considerable decrease in serum insulin, a significant increase in the fasting blood glucose concentration was observed in the untreated diabetic rats compared to healthy control, a result consistent with those reported elsewhere (21, 30, 31). Increased blood glucose concentration can be due to dysfunction of insulin secretion following STZ-induced diabetes (35). On the other hand, we found that reduction of fasting blood glucose in diabetic rats treated with the Z. officinale extract was dose-dependent, a result in accordance with those reported by several studies (21, 22, 29). The antihyperglycemic property of Z. officinal is possibly due to its phenols, polyphenol substances and flavonoids (19). Previous studies suggested that the effect of the Z. officinale extract on the reduction of blood glucose may be mediated by decreasing the absorption of glucose through inhibiting the activity of intestinal α-amylase and α-glucosidase (36), and its antagonistic activity against serotonin receptors and blocking of them (37).
Reduced blood flow into the retina is one of the most adverse consequences of the vascular dysfunction in diabetic retinopathy (1-3). Several signaling pathways are involved in the vascular dysfunction in diabetes, including, protein kinase C (PKC), endothelin, angiotensin II, and NO. Also, according to the recent studies, increased activity of arginase, induced by diabetes, plays a primary role in an impaired endothelium-dependent vasodilation response (4). Combinations of these mechanisms contribute to the development of diabetic complications (23). Previous studies conducted about the mediators, which impair retinal blood flow induced by diabetes, have demonstrated an important role for PKC and angiotensin II in changing retinal vasodilation responses. Activation of both PKC and angiotensin II pathways not only increases ROSs, but also leads to increased activity of arginase in vascular tissue. In addition, it has been showed that PKC inhibits the endothelium-dependent vasodilation of the retinal arteries. The protein kinase C can also activate the RhoA/Rho kinase signaling pathway in endothelial cells; RhoA/Rho kinase is one of the upstream regulators of arginase. Therefore, it seems that arginase is the last connector of various mediators of vascular injuries in the retina of diabetic individuals and this emphasizes the potential importance of arginase as a therapeutic target for treatment of diabetic retinopathy (4). It has been proved that oxidative stress and inflammatory reactions increase in the diabetic conditions (16). Moreover, various inflammatory factors and oxidative radicals increase arginase activity (15). Our results revealed that arginase activity and expression significantly increased in the retina of the untreated diabetic controls compared to the healthy control group, findings similar those reported by Elms et al. (4). On the other hand, our results indicated that treatment with 400 mg/kg of the Z. officinale hydroalcoholic extract resulted in a significant increase in arginase activity and expression, results which can be explained by the antiinflammatory, antioxidant and antihyperglycemic properties of the Z. officinal extract; that inhibit arginase activators (i.e. inflammatory factors, oxidative radicals and glucose). According to previous studies, Z. officinale has a compound named 6-shogaol, which possesses the most antioxidant and anti-inflammatory effects (19). On the other hand, many in vivo and in vitro studies have documented the beneficial effects of Z. officinale extract on the improvement of diabetic retinopathy (22-24).
To the best of our knowledge, our study is the first to address the effect of the Z. officinale hydroalcoholic extract on the arginase I activity and expression in the retina of STZ-induced diabetic rats. This interventional study was conducted in a sample of the STZ-induced diabetic rats; thus, clinical outcomes may be expected in further studies. However, the small sample size is a limitation in the current study and the results should be interpreted with caution. Further studies should consider replicating these results with larger sample sizes.
Conclusion: According to recent studies, the role of the arginase I has been reported in the diabetic-induced retinal vascular dysfunction (1, 10). Our results indicated that the Z. officinale hydroalcoholic extract may be a promising therapeutic potential for treating vascular disorders associated with diabetes possibly through its reducing effect on the arginase I activity and expression.