1. Introduction
Acromegaly is caused by growth hormone (GH)-secreting pituitary tumors or very rarely by extrapituitary disorders. Regardless of the etiology, the disease is characterized by elevated levels of growth hormone and insulin like growth factor 1 (IGF-I), with resultant signs and symptoms of hypersomatotrophism (1). Growth hormone-secreting adenoma may be complicated by cardiomyopathy, subclinical pituitary apoplexy and cerebrovascular disease. The presence of intracardiac thrombus was never encountered in available literature. More than rarity, the coexistence of these complications in this single patient created a therapeutic dilemma.
Cardiovascular disorders are the leading cause of morbidity and mortality in patients with acromegaly. Cardiomyopathy with congestive heart failure (CHF) is a rare complication of acromegaly occurring in less than 3% of patients (2). In one series, mortality rates were 25% at 1 year and 37.5% in 5 years. An intracardiac thrombus causing cardioembolic stroke is a possible complication of cardiomyopathy in acromegaly.
Surgery is the first line of treatment of GH-secreting tumors. However, several patients are not good surgical candidates that medical therapy becomes the primary treatment modality. Data on the effects of long-term primary medical therapy are required. We have gathered a considerable literature on the effectiveness of primary medical therapy not only with regards to tumor size and growth hormone levels but mainly on the impact of medical treatment on cardiovascular parameters.
2. Case Presentation
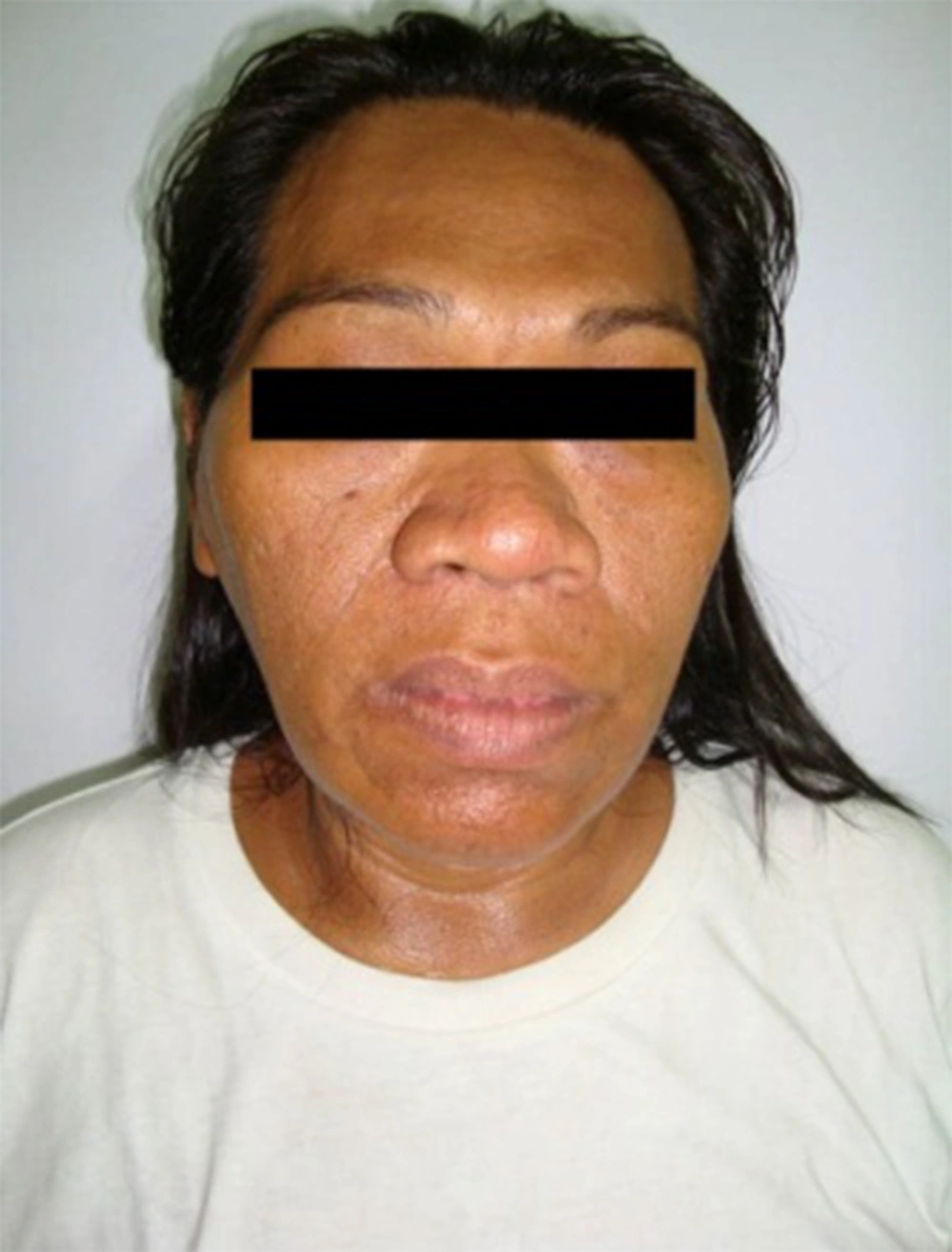
A 46-year-old Filipino woman was admitted to the hospital because of easy fatigability. She observed oligomenorrhea, decrease in libido and progressive coarsening of facial features, which started nine years ago (Figure 1). There was enlargement of digits on all extremities. Along with these clinical features, she noted progressive fatigability, orthopnea and bipedal edema. There was no headache or blurring of vision. There was no focal weakness or sensory loss. She was recently diagnosed with hypertension and diabetes mellitus. On physical examination, she has frontal bossing, nasal and mandibular enlargement, thickened lips and teeth separation. There was left ventricular heave and displaced apex beat at 6th left intercostal space anterior axillary line without any audible murmur. There is bipedal edema. On neurologic assessment, there was no visual field defect, motor and sensory loss nor sign of increased intracranial pressure.
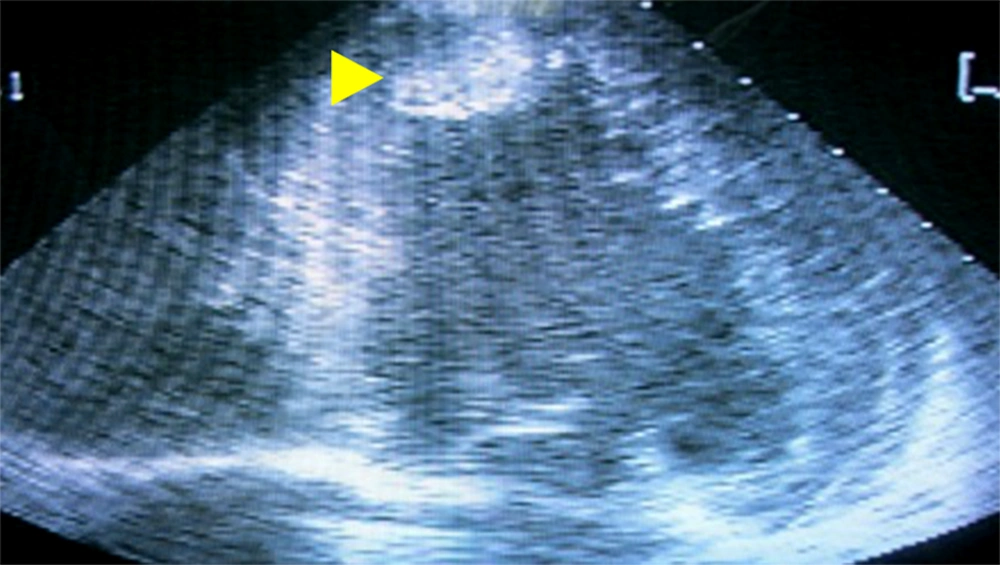
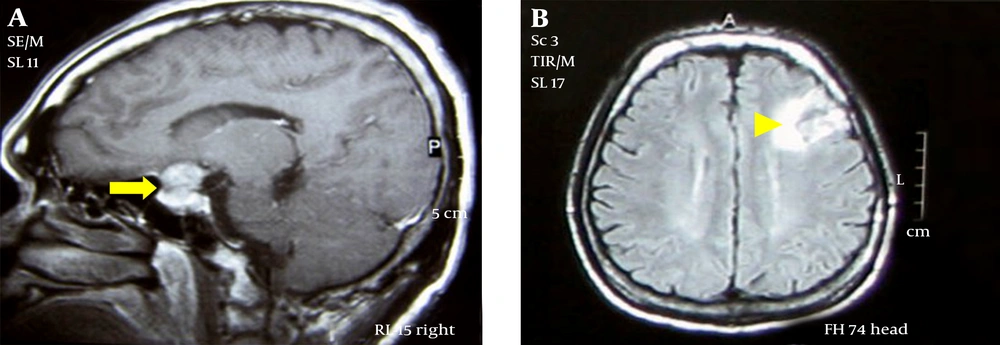
Growth hormone assay 1 hour after 75-gram oral glucose intake was elevated (> 34.90 μg/L). Serum prolactin was elevated (38.57 μg/L in 1:100 dilution). Serum TSH, free T3 and free T4 were normal. Fasting blood sugar was 9.3 mmol/L and glycosylated hemoglobin (HbA1C) was 9.86%. Left atrial enlargement and left ventricular hypertrophy were apparent on the electrocardiogram tracing. Severe systolic dysfunction (22% ejection fraction) was seen in the echocardiogram (Figure 2). Pituitary macroadenoma was seen in the Magnetic Resonance Imaging (MRI) with foci of intratumoral hemorrhages suggestive of subclinical pituitary apoplexy. Furthermore, there were subacute to chronic subcortical infarcts with hemorrhagic foci in the left frontal lobe (Figure 3).
The patient has a very high risk of developing perioperative complications because of her poor cardiovascular status. Anticoagulation, though indicated, was temporarily withheld due to the pituitary and cerebral hemorrhages. Subclinical pituitary apoplexy was managed conservatively with steroids. Primary medical therapy is most appropriate in this patient after multidisciplinary discussion. Based on current evidences available, Somatostatin Receptor Ligands (SRLs) are the first line medical treatment for GH-secreting pituitary adenomas. Dopamine agonists, however, can be an alternative considering the issues of availability and cost.
Cabergoline 0.5 mg tablet once a week was started. After 6 weeks of treatment, growth hormone level (after 75-gram oral glucose) decreased to 20.6 μg/L despite no appreciable decrease in the size of the pituitary mass. There was no deterioration of cardiovascular function. The addition of SRL octreotide to cabergoline is highly considered on follow-up of this patient.
3. Discussion
Cardiovascular disorders are the leading cause of morbidity and mortality in patients with acromegaly. Hypertension is the most frequent cardiovascular abnormality in acromegalic patients, with prevalence ranging from 15-50%, followed by atherosclerosis and coronary artery disease, which are approximately 10% of patients. Acromegaly has several effects on cardiac morphology and function that are independent of the coexistence of hypertension, suggesting the existence of a specific acromegalic cardiomyopathy (3).
Acromegalic cardiomyopathy is characterized by biventricular hypertrophy. The pathogenesis includes either direct action of GH and IGF-1 on the heart, or indirect mechanisms by which GH and IGF-1 induce hypertension and glucose intolerance with consequent increase in cardiovascular risk and remodeling. Initial cardiac involvement includes myocardial hypertrophy, but may later progress to dilated hypokinetic cardiomyopathy with left ventricular systolic dysfunction and left ventricular ejection fraction less than 45%. Congestive heart failure is a rare complication of disorders. Myocardial remodeling, hypertrophy and fibrosis all are likely to play a role in their onset. In the latter stages, acromegalic cardiomyopathy manifests as CHF with echocardiography acromegaly occurring in less than 3% of patients. A number of cardiovascular parameters improve during effective treatment of acromegaly. In general younger patients and patients with a relatively short history of acromegaly show significant recovery. In contrast, when dilated CHF occurs, cardiac function may show a short-term improvement but the longer-term prognosis is worse than that of patients with heart failure due to other causes. Hence, the major determinant of cardiomyopathy is disease duration. In one series, mortality rates were 25% at 1 year and 37.5% in 5 years (3).
Surgery is worldwide considered the first line of treatment of these primary tumors. Disease control is achieved in most microadenomas and enclosed macroadenomas. Although pituitary adenoma is potentially curable by surgery, the patient has very high risk of perioperative morbidity and mortality. Primary medical therapy is highly considered in patients with high surgical risk, extensive tumors with low likelihood of cure and patient preference (4, 5).
Somatostatin receptor ligands or somatostatin analogs (SSAs) are the most studied drugs achieving biochemical control in about 70% of patients and halts tumor growth or even causes tumor regression in about 50% (6). Somatostatin analogs therapy induces long-lasting disease control and improvement of cardiovascular risk factors, such as insulin sensitivity and high-density lipoprotein cholesterol levels in responsive patients (1). Data suggest that suppression of basal or glucose-suppressed GH levels by octreotide, together with normalization of plasma IGF-I levels for 1 year are followed by a significant improvement, but not complete normalization, of left ventricular ejection fraction either at rest or at peak exercise without significant changes in diastolic filling (7). In another retrospective study, 12 months after first-line treatment with SSA or surgery, we found a significant improvement in left ventricular hypertrophy and diastolic filling. In contrast, systolic function improved more evidently in SSA-treated patients and all arrhythmias disappeared in SSA-treated patients. The improvement was attributed either to the direct effect of SSAs or to the more preserved pituitary function (8). In a meta-analysis by Maison et al. SSA treatment was associated with significant reductions in the heart rate, left ventricular mass index and interventricular septum thickness. It was also associated with improved exercise tolerance. Trends toward beneficial effects for the left end-diastolic dimension and the left ventricular ejection fraction were also noted. Greater improvement was observed in patients with younger age and larger GH and IGF-1 reductions (9). Major drawbacks of lifelong primary treatment with SSA are the high costs of the drugs and the requirement of lifelong treatment that limits compliance. In general, side effects do not limit therapy. There was no significant difference between SSA in the risk of developing new/worsening valve regurgitation or significant regurgitation after 1 year, a concern raised with dopamine agonists (10).
Dopamine agonists (DAs) bind to D2 receptors in the pituitary and suppress GH secretion in patients with acromegaly. Although trials are more favorable with SSA, the cost of the drug is an important consideration in a developing country. In the study by Sherlock et al. even relatively low doses (median 7.5 mg/day) of bromocriptine achieved GH and IGF-1 reductions of 42% and 23.6%, respectively. These changes, although not as significant as those with SSA therapy, reduces mortality rate in these patients and therefore may still be an important alternative. The second generation DA, cabergoline, has been demonstrated to be potentially more effective in the treatment of acromegaly (11, 12). In recent study, Moyes et al. found that on a median weekly dose of 1.75 mg, normalization of both IGF-1 and GH occurred in 27% of the patients (13). There is insufficient data on whether prior radiotherapy leads to a greater reduction of GH in patients receiving DA therapy. The predictive value of prolactin regarding GH/IGF-1 response to DA therapy remains controversial. Recent studies could not find an association between pretreatment prolactin and GH/IGF-1 response to DA therapy. Therefore, this patient who has only slightly elevated prolactin should not be excluded from receiving DA on the assumption that there will be limited GH/IGF-1 response (11).
GH receptor antagonist, pegvisomant, improves acromegalic cardiomyopathy by decreasing cardiac hypertrophy and enhancing diastolic and systolic function, and consequently partially or completely reversing the cardiac insufficiency. GH receptor antagonist pegvisomant induces normalization if IGF-1 secretion, reverses LV hypertrophy and improves systolic and diastolic performance (14). Pegvisomant can be an adjunct to SRLs (15). However, tumor growth is observed in 2 patients. The presumed mechanism of tumor enlargement is by loss of the inhibitory effect on tumor growth when IGF-1 levels are reduced (16).
Radiotherapy is effective in reducing GH and IGF-1 levels; however, there is often a period of several years before this effect is achieved. Of greater consideration in our patient, the use of conventional radiotherapy in patients with acromegaly has been associated with increased cerebrovascular mortality (17). The relative risk of first cerebrovascular accident (CVA) compared to the general population was 4.1. On multivariate analysis, higher administered dose of radiotherapy emerged as one of the main predictors of CVA. The relative risk of cerebrovascular deaths in patients with functioning is higher than nonfunctioning tumors (5.23 vs. 3.65) in another study by Brada et al. (18) Previous treatment with radiotherapy may induce partial resistance to medical treatment especially SRLs (19).
Although acromegaly is an insidious disease, it is associated with increased morbidity and mortality mainly because of cardiovascular complications. Cardiomyopathy with CHF occurs only in 3% of patients, but its presence may change decision in management. Medical therapy becomes the primary treatment for those who are poor surgical candidates. Aside from tumor shrinkage and reduction of GH and IGF-1, there are available evidences that treatment with SRLs can improve cardiovascular function. Dopamine agonists can still be considered regardless of prolactin level and immunostaining. Adjunctive radiation therapy must be weighed against the risk of worsening cerebrovascular disease. Given the limited options for treatment, the risks and benefits must be emphasized.