1. Background
Worldwide prevalence of diabetes has been recently increased over two-fold and is estimated to affect 439 million people by the year 2030 (1, 2). Diabetes is a complex metabolic disease characterized by high blood glucose level resulting from defects in insulin secretion or action (2). 1, 25-dihydroxyvitamin D3 (vit D), the most potent metabolite of vitamin D, plays an important role in calcium and phosphate homeostasis, also vital for a number of other biological activities including immunomodulation and normal release of insulin from β cells (3-5). Pancreatic islets have both vitamin D receptors and vitamin D-dependent calcium-binding proteins, suggesting a role for vitamin D in insulin secretion (6, 7).
It has been reported that vitamin D deficiency impairs insulin synthesis and secretion in both human and animal models and its replenishment improves β cell function and glucose tolerance (4, 8, 9). Cade et al. have shown improvement in glucose metabolism and insulin secretion in vitamin D-deficient rats following a single subcutaneous injection of vitamin D (10). In addition, it has been reported that de novo insulin synthesis is reduced in isolated islets in vitamin D-deficient rats and insulin biosynthetic capacity can be restored in vitro by injection of vitamin D (9). vitamin D deficiency may be related to type 2 diabetes, cardiovascular disease and cancer (11, 12). Subjects with type 2 diabetes have lower circulating vitamin D levels compared to healthy controls (3, 13). Furthermore, epidemiological studies have shown that vitamin D receptor restriction site polymorphisms are associated with genetic susceptibility to type 1 diabetes in different populations, while vitamin D supplementation in early childhood is associated with a reduced risk of type 1 diabetes (14). Nikooyeh et al. have reported that daily consumption of vitamin D improves glycemic status in patients with type 2 diabetes (15). Interventional studies investigating vitamin D for treatment of type 2 diabetes have shown negligible effects, and the results available in humans have been contradictory (15).
2. Objectives
Previous studies have reported that high glucose concentrations significantly decreased total insulin secretion from β cells (16). The effects of coincubation and preincubation of vitamin D with high glucose concentration on insulin secretion from islets have not been fully understood yet. Therefore, the aim of this study was to determine the effects of vitamin D on insulin release from isolated islets of rats.
3. Materials and Methods
Adult male Wistar rats (two months old, weighing 200-250 grams) were obtained from the laboratory animal house of the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences and housed in an animal room at 22 ± 3°C, relative humidity of 50 ± 6%, an inverse 12: 12 hour light/dark cycle. Rats had free access to standard rat chow (Pars Co., Tehran) and tap water during the study. All experimental procedures and rat care and handling were performed in accordance with guidelines provided by the local ethics committee of the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences.
Animals were anesthetized by intraperitoneal injection of ketamine/xylazine (50 mg/kg and 10 mg/kg) and the abdomen was exposed, and 0.5 mg/mL of collagenase P (Roche, Cat. # 1213, Germany) in 10 mL cold Hanks’ balanced salt solution (HBSS) [pH = 7.4; containing KCl, 5.36; CaCl2, 1.26; NaCl, 136; MgSO4 7 H2O, 0.8; Na2HPO4 2 H2O, 0.33; NaHCO3, 4.16; KH2 PO4, 0.44) all in mM (Merck, Germany) and gassed with 95% O2, 5% CO2 for five minutes at the beginning] was injected into the common bile duct; the expanded pancreas was detached, transmitted to a 50 mL falcon container and placed in a water bath (37°C) for 15 minutes. At the end of digestion, 30 mL cold HBSS was added and the tube was shaken for one minute. Tube contents were filtered through a 500 μm plastic mesh to discard any undigested tissue. After three washes with cold HBSS, islets were selected under a stereomicroscope (Kyowa, SDZ-TR-PL Japan); a group of 10 islets were incubated in a cell strainer containing 4 mL of RPMI 1640 medium containing 10% fetal bovine serum and 1% of penicillin/streptomycin (17, 18). Insulin release was assessed in response to vitamin D (D1530, Sigma) following 24 and 48 hours coincubation of islets with vitamin D (0.1, 1, and 10 nM) and glucose (5.6, 11.1 and 16.7 mM).
In addition, islets were preincubated with vitamin D for 24 and 48 hours and glucose-stimulated insulin secretion (GSIS) was assessed for one hour in the presence of 5.6 and 16.7 mM glucose. For in vitro assessment of insulin secretion, batches of eight islets were transferred into 1.5 mL plastic cups, and 1 mL of Krebs Ringer solution [(pH = 7.4); NaCl, 115; KCl, 5; MgCl2 6 H2O, 1; CaCl2 , 2.5; NaHCO3, 24 (Merck, Germany); HEPES, 16 (Sigma, USA) all in mM] and 5 g/dL BSA (Fluka, USA) with different glucose concentrations (5.6 and 16.7 mM) were added into the cups, incubated for 60 minutes in 37°C water bath and gassed with 95% O2, 5% CO2 for five minutes at the beginning. Aliquots of supernatant were collected and stored at -20°C for insulin determination. Insulin concentration was measured by ELISA technique (Mercodia, Sweden). Intra and inter-assay coefficients of variations for insulin measurements were 6.1% and 9.7%, respectively.
3.1. Statistical Analysis
All data were expressed as mean ± SEM and analyzed using GraphPad Prism software (Version 5.00 for Windows, GraphPad Software, San Diego California USA). Two-way analysis of variance (ANOVA) and Bonferroni post-hoc test were used to compare serum insulin concentrations in different levels of glucose and vitamin D. P value below 0.05 was considered statistically significant.
4. Results
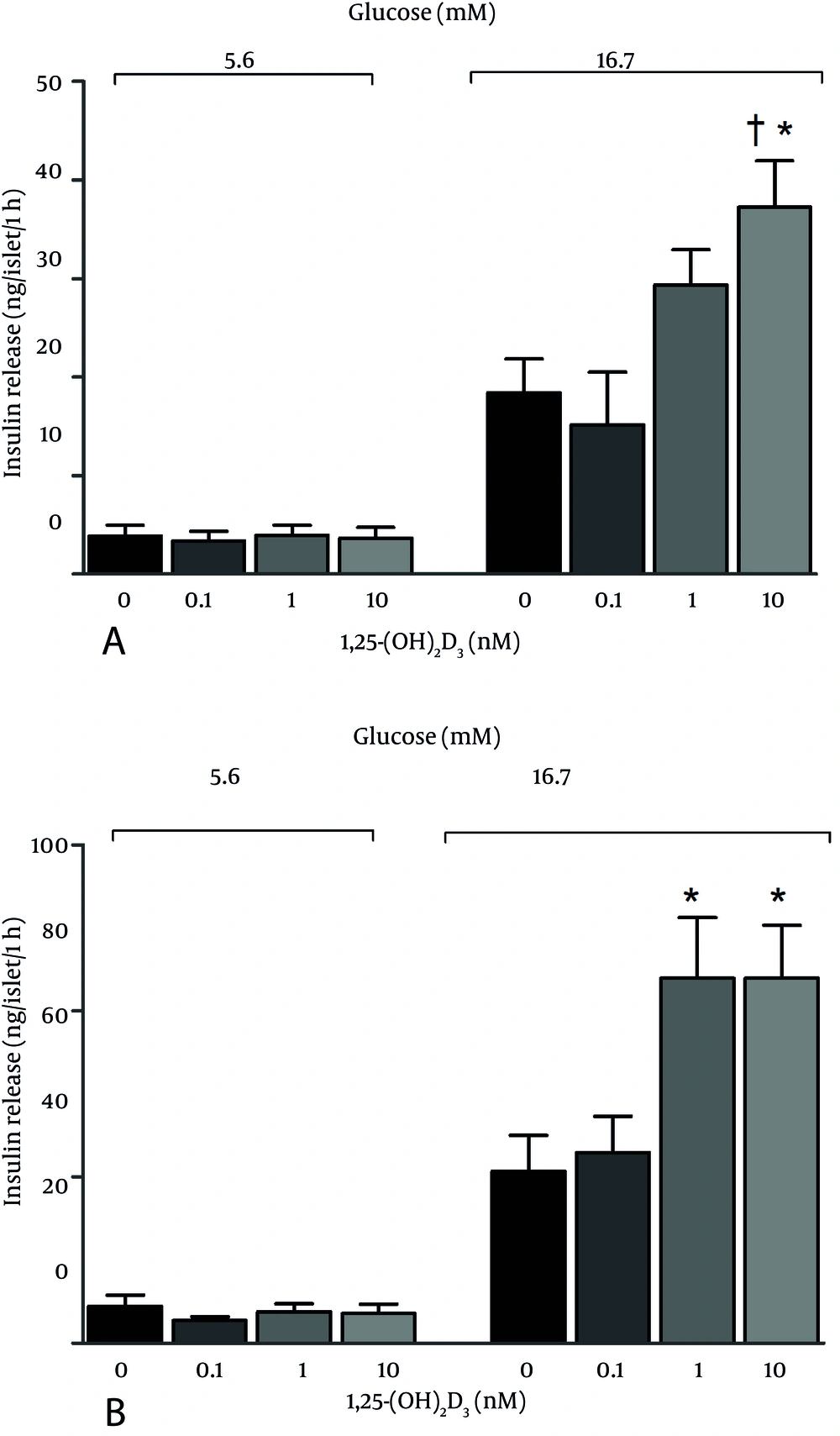
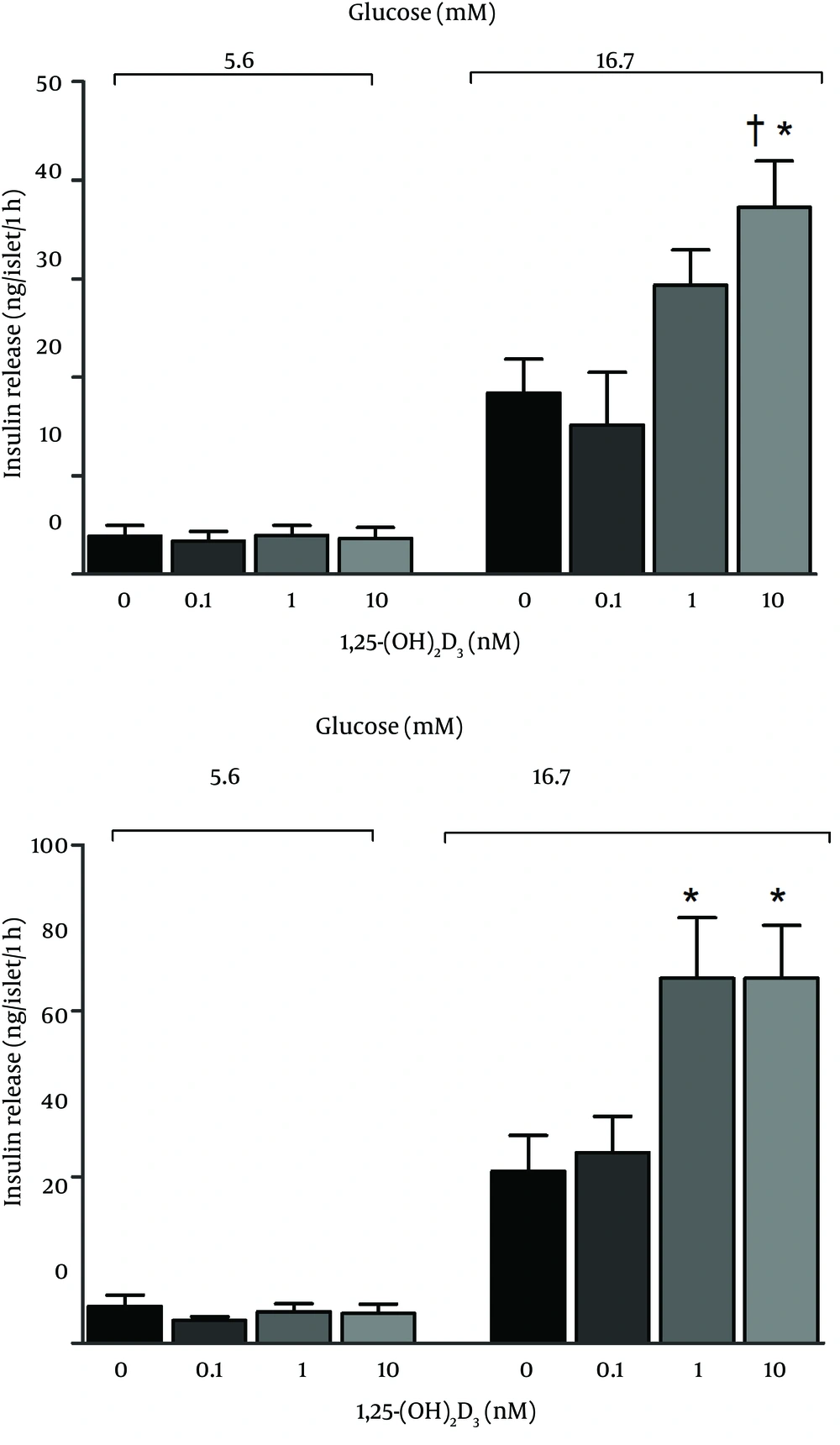
Effects of coincubation of vitamin D with glucose on insulin release from islets were shown in Figure 1. As shown, coincubation of islets with 10 nM of vitamin D and 11.1 mM glucose increased insulin release, while 1 and 10 nM vitamin D with 16.7 mM glucose decreased insulin release. Vitamin D had no effect on insulin release from islets during 24 hours coincubation with 5.6 mM glucose or 48 hours Coincubation with 5.6, 11.1, and 16.7 mM glucose. Effects of 5.6 and 16.7 mM glucose on GSIS in islets, following 24 and 48 hours preincubation with vitamin D were shown in Figure 2. As seen, 24 and 48 hours preincubation with 10 nM of vitamin D and 48 hours preincubation with 1 and 10 nM of vitamin D increased GSIS in the presence of 16.7 mM glucose. No significant changes were detected in response to 5.6 mM glucose.
5. Discussion
Preincubation of vitamin D with glucose for 24 or 48 hours increased GSIS from islets only at high doses of vitamin D and glucose. In addition, when vitamin D and glucose were coincubated for 24 hours, insulin release was increased with 10 nM vitamin D and 11.1 mM glucose; whereas, insulin release decreased with 1 and 10 nM vitamin D and 16.7 glucose. These results were consistent with those studies reporting an association between vitamin D and insulin release from β cells (19-21).
Preincubation with vitamin D increased GSIS in response to 16.7 mM glucose, findings in agreement with the results of Billaudel et al. who reported that GSIS is eliminated by vitamin D deficiency in rats; however, six hours preincubation with 0.001-1000 nM vitamin D had a stimulatory effect on GSIS in response to 8.3 mM glucose (8). Furthermore, it has been reported that 24 hours preincubation with 10 nM vitamin D increases GSIS in the presence of 8.3 mM glucose in vitamin D-deficient rats (22). Tanaka et al. have also reported that insulin secretion induced by 16.7 mM glucose in vitamin D deficient rats was only 35% of that of control rats, but was restored to that of the controls in vitamin D-treated rats (23). Bourlon et al. have also reported that insulin secretion is destroyed in vitamin D deficient rats and restored by 1, 25-(OH)2D3 (24).
Results of this study showed that vitamin D affects insulin secretion only in the presence of high glucose level, whereas it does not affect insulin secretion in presence of 5.6 mM glucose. Vitamin D enhances sensitivity of β cells to glucose, and has been suggested that the effect of vitamin D on insulin secretion may be ATP-dependent (4, 22). Consistent with our results, d’Emden et al. have shown that coincubation of 0.1 nM and 1 nM vitamin D with 10 mM glucose did not increase insulin release in medium for up to 180 hours (25). Faure et al. have confirmed that coincubation of 0.001 nM vitamin D with 8.3 mM glucose did not affect insulin secretion (22). Chan et al. have reported that in vitro addition of vitamin D had no effect on insulin secretion from normal or vitamin D deficient rats (26). In addition, two human studies found no change in insulin release following vitamin D supplementation in adults with either obesity or insulin resistance (19-21). Contradictory to our results, Billaudel et al. have reported that coincubation of 0.001-1000 nM of vitamin D with 8.3 mM glucose had an incremental dose-dependent effect on insulin secretion (8). Moreover, d’Emden et al. Have demonstrated that coincubation of 10 nM vitamin D with 10 mM glucose increased insulin secretion 2.5 fold in a β cell medium (25).
To date, no study has investigated the effect of vitamin D on insulin secretion in the presence of 16.7 mM glucose in short duration (up to 24 hours). Lower insulin secretion has been reported in the presence of high glucose concentrations compared to low glucose concentrations in β cell medium. In addition, high glucose concentrations significantly decrease insulin secretion in cultured β cells; these data indicates that exposure to high glucose levels induces a glucotoxic situation, which could impair insulin secretion in response to glucose (16). Contrary to the finding of our study, d’Emden et al. have demonstrated that coincubation of 10 nM vitamin D with 20 mM glucose increased insulin secretion in a β cell medium (25), but suggested a significantly increased insulin release in islets following 96 hours coincubation or longer.
The exact mechanisms of vitamin D effect on insulin secretion in the presence of glucose are not yet fully understood and need further studies, but it has been suggested that vitamin D directly increases insulin secretion in β cells (25). Faure et al. have reported that vitamin D enhances Ca2+ entry or Ca2+ mobilization in β cells, which could amplify insulin secretion in the presence of glucose (22). Also Bourlon et al. have also shown that vitamin D requires a delay to increase insulin secretion from β cells; this delay varies from 6 hours pre-incubation in vitro, 3 hours to 20 hours in vivo and 48 hours in culture, which can indicates the genomic effects of vitamin D (9).
In conclusion, effects of coincubation of islets with vitamin D and glucose depend on glucose concentration. Vitamin D significantly decreased insulin secretion in the presence of 16.7 mM glucose, but increased insulin secretion in the presence of 11.1 mM glucose. In addition, this study showed that preincubation of islets with vitamin D increased GSIS at high levels of glucose (16.7 mM), while no effect was observed at low levels.