1. Background
Mini-puberty is the term used to refer to the transient surge in the hypothalamic gonadotropin-releasing hormone (GnRH) pulse generator (pituitary gonadotropin) gonadal apparatus after birth. Due to the sudden drop of the mother’s sex hormones, male baby’s gonadotropin levels increase from the first week after birth and peak at the third month, followed by testosterone (1). This period is thought to be associated with the special secretion of many sex hormones and is an important stage in sexual development (2, 3). Because of the same secretion pattern of the luteinizing hormone (LH), follicle-stimulating hormone (FSH) and testosterone (T) at puberty time, the hormone levels decrease during this period may indicate the fail of hypothalamus-pituitary-gonad (HPG) axis. Studying the existence of mini-puberty can give us a hint of HPG axis function in early infancy; furthermore, mini-puberty is thought to be important for testicular function later in life (4). A previous study confirmed the existence of a six-hour mini-pubertal period in neonatal male rats (5). In this study, we attempted to establish a “no mini-puberty” model using male rats and investigate the importance of mini-puberty in testicular function through serum sex hormone levels, the expression of related genes, and the morphologic alterations in the testis.
Mammalian testes are composed of seminiferous (containing germ and Sertoli cells) and interstitial tissue (containing Leydig cells) (6). Normal testis development is under the regulation of LH and FSH secreted by pituitary gland (7). Besides, many regulatory factors or sex hormones secreted by testis can reflect its function and regulate the development of itself, such as anti-Mullerian hormone (AMH), insulin-like growth factor 3 (INSL3) and ghrelin. A research (8) suggested that Sertoli cells kept proliferating during the whole prepubertal time due to the high level of serum AMH concentration. AMH is a distinctive marker of Sertoli cells; it is synthesized by Sertoli cells and basal AMH expression is independent on gonadotropin stimulation (9). Ghrelin is expressed in interstitial mature Leydig cells of the human testis (10). It is an important regulator of the Leydig cells function (11). Growing evidence indicates that ghrelin plays a role in the control of reproductive function and fertility. INSL3 is a major secreted product of the Leydig cells of the fetal testes and also acts as an important indicator of the Leydig cells function (12-14). It is thought to serves as an excellent marker for Leydig cells differentiation and functional capacity (15). Research showed that INSL3 was mostly synergizing with gonadotropin-induced androgens action (16, 17). Androgens are responsible for male testicular development during embryogenesis, puberty and the maintenance of male reproductive capacity as well as the behavior in adulthood (18). Their function is dependent on signaling through the androgen receptor (AR), which is expressed in both spermatogenic cells and Leydig cells.
Considering the key roles played by AMH, ghrelin, INSL3 and AR during the process of male testicular function, we tested the mRNA levels of the four genes in the rat models as well as their hormone levels, testicular weights and morphologic alterations, to investigate the effect of hormone inhibition during the mini-puberty period on testicular function in male rats and to compare the extent of the damage between Leydig cells and Sertoli cells.
2. Objectives
This study aimed to establish a “no mini-puberty” model using male rats and compare changes of serum sex hormone levels, the related gene expression and morphologic alterations in the testis to investigate the importance of mini-puberty in testicular function.
3. Materials and Methods
3.1. Experimental Animals
Clean-level female pregnant Wistar rats were purchased from Lake Hayes Animals LLC (License No. SYXK (Shanghai), 2012-0002). The animals were housed separately and given standard food until delivery. Thereafter, male pups were selected at different postnatal times according to the requirements of the experiment. Some pups were also raised to adulthood as required by the experimental protocol.
3.2. Methods
The serum levels of testosterone (T), LH and FSH were determined using commercial direct enzyme-linked immunosorbent assay (ELISA) kits (rat T ELISA kit, DEV9911, Demeditec, Germany; rat LHELISA kit, AKRLH-010, Shibayagi, Japan; rat FSH ELISA kit, AKRFS-010, Shibayagi, Japan). The testicular wet weight of the normal male offsprings were measured at different time points after birth (0, 2, 4, 6, and 24 hours) by an electronic analytical balance (Shanghai Precision and Scientific Instrument Corporation, China; measuring sensitivity: 0.0001 g). There were eight rats for each time point.
The analyses of the reproducibility of the indirect ELISAs showed the following: the rat testosterone ELISA kit sensitivity was 0.066 ng/mL with a coefficient of variation (CV) of 6.5 ~ 11% within plates and 9.3 ~ 11% between plates; the rat luteinizing hormone ELISA kit determination range was 0.313 ~ 10 ng/mL, while the CV was < 5% both within plates and between plates; the rat follicle-stimulating hormone ELISA kit determination range was 0.4 ~ 20 ng/mL with CV < 5% within plates and between plates. There were three runs of each hormone assay in both groups.
Normal male rat offsprings were randomly selected as the model group and placed in an airtight container containing ether-soaked cotton for five minutes after birth until spontaneous activity stopped (average time: approximately eight minutes) and the rats were then returned to their mother. The rats from the control group were placed in an airtight container without ether. Six rats from each group were sacrificed by cervical decapitation when the serum sex hormones reached a transient surge (about two hours after birth) and blood samples were obtained for testing the serum T, LH, and FSH concentrations.
On postnatal day 45 (PND45) when the rats attained puberty, six male rat offsprings were randomly selected from each of the model and control groups. The wet weights of their bilateral testes, epididymes and bodies were obtained. The serum T levels were determined by ELISA kits. Testicular tissue biopsies were embedded in paraffin and sliced into 5-µm thin cross-sections for hematoxylin and eosin (H & E) staining. Finally, we quantified the mRNA expression levels of testicular AR, INSL3, AMH and ghrelin using real-time polymerase chain reaction (PCR) (ABI Prism 7300 Sequence Detection System, Applied Biosystems). Master mix was SYBR Premix Ex Taq (Perfect Real Time) (TaKaRa), and RNA was extracted by Trizol (Invitrogen Corporation). The tests were Triplicate. Reference gene was β-Actin and data was relative.
When the rats attained sexual maturity on PND75, six male rat offsprings were randomly selected from each group and the same parameters were measured, as described before.
3.3. Statistical Analysis and Ethical Consideration
All the data were expressed as the mean ± standard deviation (SD). Data were analyzed using statistical package for social sciences (SPSS) version 16.0. Data were compared using one-way analysis of variance (ANOVA), followed by Student’s t-test. Statistical differences were considered significant when P < 0.05. This animal experiment was approved by Animal Ethics Committee.
4. Results
4.1. Mini-Puberty
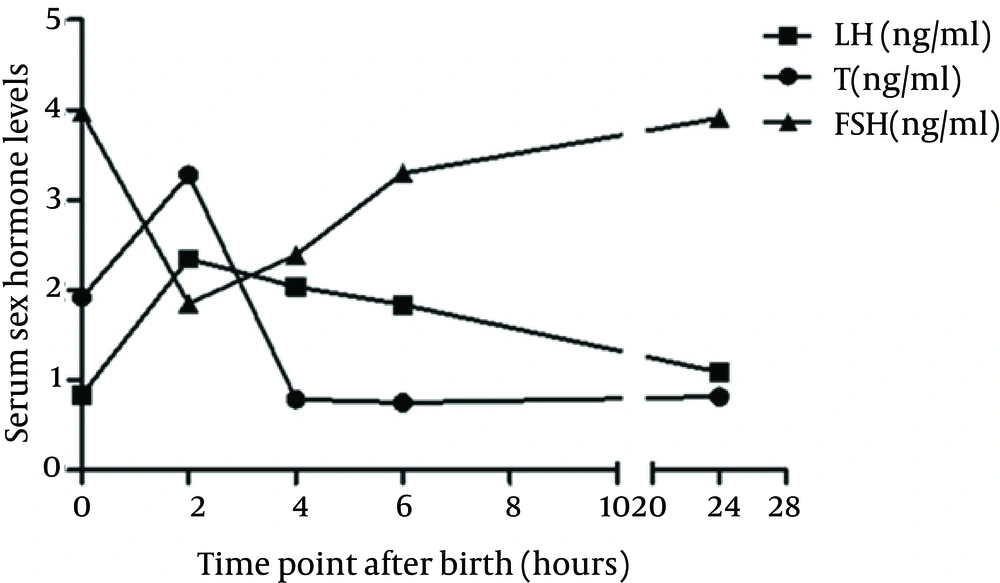
Table 1 shows the serum T, LH, and FSH levels in male pups at different time points after birth (0, 2, 4, 6, and 24 hours). As shown in Table 1 and Figure 1, the serum T and LH levels started to increase immediately after birth, peaked at two hours after birth, and declined within about six hours. Meanwhile, serum FSH levels peaked immediately after birth, reaching their lowest levels at two hours, before slowly elevating throughout the next three time points. Table 1 also presents the changes in the wet weights of the bilateral testes and epididymes at different time points after birth. The wet weights of both the organs as well as their ratios to the body weight increased to a transient peak at the forth hour.
| Variables | Time Point After Birth (hours) | ||||
|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 24 | |
| T, ng/mL | 1.92 ± 0.69 c | 3.28 ± 1.04 | 0.79 ± 0.17 | 0.75 ± 0.53 c | 0.82 ± 0.24 |
| LH, ng/mL | 0.84 ± 0.47 c | 2.35 ± 0.49 | 2.04 ± 0.79 | 1.84 ± 1.02 c | 1.09 ± 0.47 |
| FSH, ng/mL | 3.97 ± 0.57 | 1.85 ± 0.65 d | 2.39 ± 0.71 | 3.30 ± 0.99 d | 3.91 ± 1.21 |
| Bilateral testes and epididymis weight, g (× 10-2) | 0.37 ± 0.11 | 0.39 ± 0.15 | 0.55 ± 0.08 | 0.45 ± 0.03 | 0.52 ± 0.1 |
| Ratio of bilateral testes and epididymis weight to body weight (× 10-2) | 0.07 ± 0.02 | 0.08 ± 0.03 | 0.09 ± 0.01 | 0.08 ± 0.002 | 0.08 ± 0.01 |
4.2. Establishment of a “No Mini-Puberty” Model
Table 2 shows the serum T, LH, and FSH levels in neonatal male rats of the model and control groups. Data revealed a significant decline (P < 0.05) in the serum T, LH, and FSH levels using ether inhalation, indicating the establishment of a “no mini-puberty” model.
4.3. Comparison of the Activity of the Hypothalamus-Pituitary Gland-Gonad Axis Between the Model and Control Groups
There were no significant differences in the bilateral testicle and epididymis weights between the model and control groups on PND45 and PND75 (P > 0.05). Similarly, there were no significant differences in the serum T levels between the two groups on PND45 and PND75 (P > 0.05).
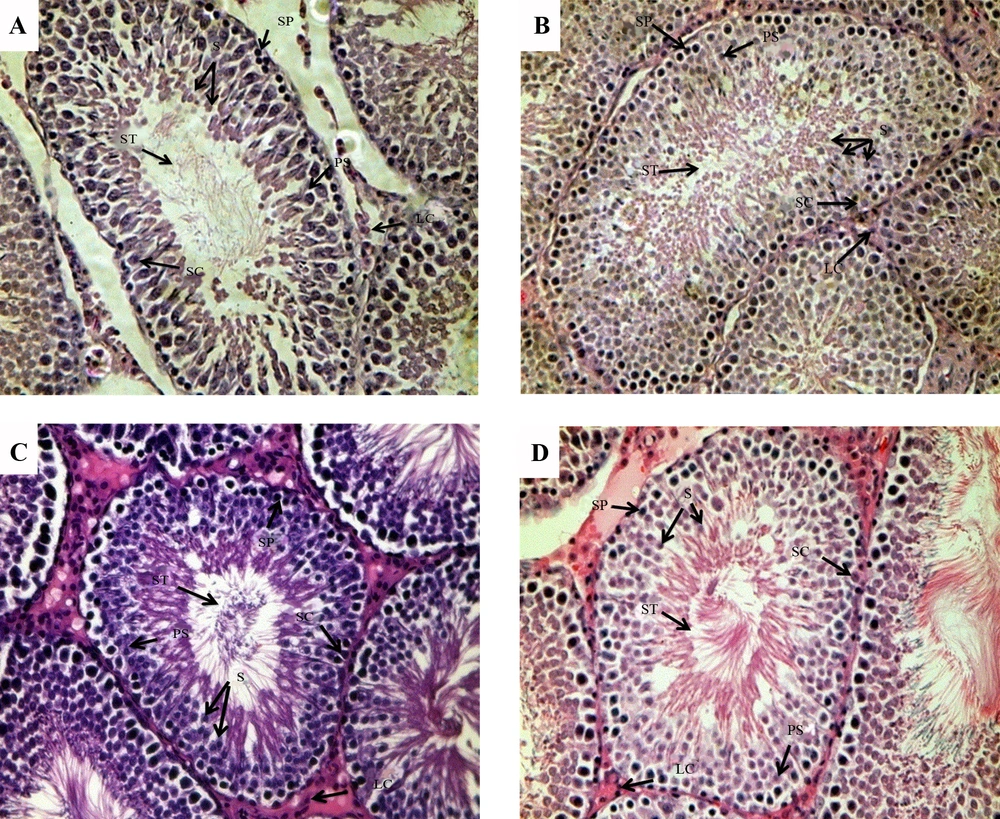
Images of H&E staining (Figure 2) illustrates the morphologic changes in the testes. On PND45, the layer of spermatogenic epithelium, including spermatogenic cells and Sertoli cells both decreased in the model group. Spermatogenic cells were loosely packed and the cells number of all the stages of spermatid differentiation decreased significantly compared to the control group, whereas cells in the control group had normal morphologies and were well aligned. Furthermore, there were no significant differences in the cell morphology on PND75 between the two groups.
The mRNA expression levels of testicular AR, INSL3, AMH, and ghrelin were quantitatively analyzed by real-time PCR (Table 3). On PND45, the ghrelin mRNA level showed a decreasing trend in the model group compared with the control (P = 0.055), but the AR, INSL3, and AMH mRNA levels showed no significant differences between the two groups. On PND75, the AMH mRNA level significantly decreased in the model group compared to that of the control group (P < 0.05) and the AR of the mRNA group also decreased in the model group (P = 0.05). There were no significant differences in the INSL3 and ghrelin mRNA levels between the two groups.
| Variables | Model on PND 45 | Control on PND 45 | Model on PND 75 | Control on PND 75 |
|---|---|---|---|---|
| AR | 1.361 ± 0.361 | 1.433 ± 0.449 | 1.408 ± 0.118 | 1.746 ± 0.178 |
| INSL3 | 0.892 ± 0.216 | 1.032 ± 0.059 | 0.392 ± 0.05 | 0.498 ± 0.063 |
| Ghrelin | 0.546 ± 0.186 | 0.903 ± 0.136 | 0.814 ± 0.369 | 0.835 ± 0.39 |
| AMH | 1.412 ± 0.194 | 1.418 ± 0.139 | 0.424 ± 0.099 c | 0.632 ± 0.024 |
5. Discussion
We chose Wistar rats to confirm the existence of mini-puberty and to investigate the impacts of hormone inhibition during this phase on adolescent gonadal axis function. The concentrations of serum sex hormones revealed a transient surge, lasting about six hours, in the hypothalamic–pituitary–gonadal (HPG) axis of male pups; these findings were similar to that reported in Corbier’s study (5). Meanwhile, the testis weight peaked at four hours after birth following the surge of sex hormones; this may be caused by the increasing effect of sex hormones on neonatal testis tissue. As reported by Svechnikov (11), postnatal differentiation of testicular cells (including Leydig cells, Sertoli cells and spermatogenic cells) involved a variety of cellular cascades, resulting in mature testicular cells with well-developed steroidogenic machinery required for testosterone biosynthesis and adolescent gonadal axis function. There are two periods of activities in the HPG axis before the onset of puberty: the first during the fetal life and the second during the postnatal months in human (19). After the postnatal HPG axis activity, the axis is silenced for several years until puberty. Therefore, mini-puberty provides a window of opportunity to study the normal mechanisms that occur from prepuberty to sexual maturity and to get an early assessment of testicular function.
In mammals such as rats, the regulation of testicular function, fundamentally involving complex developmental genetics and endocrinology, can be subdivided into chromosomal, genetic, and hormonal stages, where each stage influences the next, resulting in not only sex differences in the reproductive system but also sexual differentiation of the central nervous system (CNS) (20). The brain is particularly sensitive to the differentiating effects of androgen only during a critical period of exposure to the supraphysiological androgen levels (21). Lamminmaki (22) has found early postnatal testosterone levels showed predicted associations with later behavior after measuring testosterone in 48 full-terminfants by monthly urinary sampling from day seven postnatal to six months old and analyzing their sex-typed behavior. In our study, the serum T levels peaked at two hours, reaching a value of four times of that at birth; this may indicate that the sudden secretion in the neonatal period plays an important role in CNS sexual differentiation as well as the masculinization process at adulthood.
Vega (23) observed that the postnatal T surge was inhibited in male rat pups anesthetized with ether inhalation immediately after delivery, and that this inhibition resulted in permanent alteration of adulthood sexual behavior, as the male rats showed elevated levels of feminine behaviors and impaired masculine sexual behaviors. However, ether inhalation anesthesia performed at four hours after birth resulted in no significant impact on adulthood sexual behavior in the rats. These findings suggest that the postnatal T surge may be involved in sexual differentiation of the CNS and influence the masculinization processes. Arena (24) investigated the effects of ether inhalation on male rats immediately after birth and found alterations in sexual behavior along with a decrease in the number of spermatids and spermatozoa at adulthood, which suggested that ether delayed or reduced the testosterone peak of the sex differentiation period, consequently altering the processes of masculinization and defeminization of the CNS.
On the basis of the above observations, we investigated the long-term effects of hormone inhibition during the mini-pubertal period on testicular function in male rats at a molecular and morphologic level. In the present study, the levels of serum T, LH, and FSH concentrations in the model group were significantly lower than the corresponding levels in the control group, indicating that ether inhalation was an inhibitory factor for pituitary gonadotropin secretion and reduced T surge, which in turn means that mini-puberty was inhibited in the model mice. The morphological analysis of the testes on PND45 revealed damage of spermatogenic cells in the model mice. The ghrelin mRNA expression level showed a decreasing trend in the model group compared with the control group. Evidence supports that ghrelin is expressed and operates at different levels in the gonadotropic axis and in other reproductive tissues (10). It is also involved in the secretion of gonadotropin and prolactin and is highly selectively expressed in interstitial mature Leydig cells of the human testis. Therefore, the decreased expression level may indicate a degree of damage of Leydig cells and their function. On PND75, there was a significant decrease in the AMH mRNA expression level as well as a decreasing trend of the AR gene. As AMH is produced by Sertoli cells and is responsible for the regression of the Mullerian ducts, the anlagen of the uterus and Fallopian tubes (9, 25), it is an excellent marker of Sertoli cells function. Therefore, the significant decrease in the AMH mRNA expression level indicated damage to testicular Sertoli cells function in our adult male rat model. A research (9) has shown that seminiferous tubules are the major components of the testis, while seminiferous tubule volume depends mainly on Sertoli cells during the whole prepubertal period, especially during mini-puberty; thus, hormone inhibition during mini-puberty can be expected to mainly affect Sertoli cells, which is consistent with our results. Acting through AR, androgens are responsible for the development of the male phenotype and for male sex maturation and the maintenance of male reproductive function and behavior (18). The decrease in the AR mRNA expression level suggests that the masculinization of the model rats in our study may be partly damaged. INSL3 is a major secretory product of Leydig cells (26) which reflects their number, differentiation status and ability to produce various factors including steroids. INSL3 is not acutely regulated by the HPG axis (15), making it an excellent marker for Leydig cells differentiation and functional capacity. INSL3 is important for the process of testicular descent, specifically in transabdominal testis translocation (27, 28). However, in our research, we did not find any significant differences in the INSL3 mRNA level on PND45 and PND75, suggesting that hormone inhibition in neonatal rats does not get its long-term effects through INSL3. The testicular normal development relies on many regulatory factors and sex hormones, along with their interactions and balance. The decrease of mRNA expression of ghrelin, AMH, AR and INSL3 indicated the damage of the function of Leydig cells and Sertoli cells at different periods and that the balance between them may be broken.
The fact that inhibition in the transient hormone surge of the HPG axis in neonatal male rats had a long-term effect on testicular function at puberty and sexual maturity, confirmed that mini-puberty is a special phase in the male sexual defeminization and masculinization process. The optimized therapy of sexual disorders, especially of congenital hypogonadotropic hypogonadism remains to pose a challenge to pediatric endocrinologists, which may partly be caused by an incomplete medication treatment plan; but, delays in diagnosis should be taken seriously. As mini-puberty has been found to play a vital role in the testicular function, it is meaningful to place a high value on this period to establish an early diagnosis of hypogonadotropic hypogonadism, so that it can be treated at an appropriate time.