1. Background
Metabolic syndrome (MetS) has become a major public health concern worldwide because of its associations with increased risk of type 2 diabetes (T2DM), cardiovascular morbidity and mortality and all-cause of mortality (1, 2). MetS prevalence is increasing rapidly due to urbanization and its consequences on life styles and dietary food habits (1, 2). The Mediterranean diet (MedDiet), as a model of healthy pattern, has been suggested to play a beneficial role in the prevention of cardiovascular disease, cancer, T2DM and all-cause mortality (3, 4). Most previous studies reported the possible protective effect of adherence to the MedDiet on MetS prevalence and its progression (5, 6); however, most investigations had preliminary cross-sectional designs (5, 6), and only three studies investigated prospectively the association between adherence to the MedDiet and development of MetS (7-9). In addition, most current knowledge on the influence of the MedDiet on the risk of MetS comes from studies conducted among Mediterranean populations and therefore the benefits observed could be attributed to other potential confounders such as genetic and environmental factors (10). Furthermore, studies investigating the association between the pattern and each individual MetS components provided inconsistent findings (7-9, 11, 12). It is important to assess the association between adherence to the MedDiet and health outcomes in non-Mediterranean populations, because there is an interest in encouraging people with different cultures to adopt the MedDiet for primary and secondary prevention of chronic diseases.
2. Objectives
Considering the high prevalence and incident MetS in Iranian population (13, 14), we aimed to prospectively examine the relation of adherence to the MedDiet with 1) mean values of MetS components 2) incidence of abnormalities in each MetS component and 3) MetS incidence in non-diabetic Iranian populations after 3 years of follow-up using two different the MedDiet scores.
3. Patients and Methods
The Tehran lipid and glucose study (TLGS) is an ongoing urban-population based prospective study aimed at identifying and preventing non-communicable diseases in district No. 13 of Tehran, the capital city of Iran. The initial TLGS study population consisted of 15005 (women and men, aged ≥ 3 years), recruited during 1999 - 2001. Participants are evaluated every 3 years to update health-related data and identify newly developed diseases (15, 16). The study was approved by the ethics committee of the Research Institute for endocrine sciences of Shahid Beheshti university of medical sciences and written informed consent was obtained from each participant.
For the current study, we used data collected at the third examination (2005 - 2008) as baseline and that of the fourth examination (2008 - 2011) as follow-up. Of 12523 individuals who completed the third examination, 4920 were randomly selected for dietary assessment, based on their age and sex and 3687 completed the dietary assessment. Characteristics of participants who completed the dietary assessment were similar to those of the total population in the third examination of TLGS (17). Among the participants, 3133 individuals aged 18 - 74 years were selected for the current study. Participants with implausible values for total energy intake at baseline (n = 110), those with missing baseline (n = 82) or follow-up components of MetS information (n = 531) and those with diabetes (n = 169) were excluded. After exclusions, 2241 participants remained for the longitudinal study of the association between the MedDiet scores and mean values of MetS components. For the analysis of the incidence of abnormalities in each MetS component, the number of participants was 1123 for high waist circumference (WC), 2045 for high fasting plasma glucose (FPG), 1530 for high triglycerides (TG), 817 for low high-density lipoprotein (HDL-C) and 1837 for high blood pressure (BP) after exclusion of prevalent abnormality in each component at baseline. For the analysis of the MetS incidence, 1661 participants remained after exclusion of individuals with MetS at baseline (n = 580).
3.1. Assessment of Adherence to the Mediterranean Diet
A valid and reliable food frequency questionnaire (FFQ) of 168 food items with standard serving sizes was used by trained dietitians to assess the usual food intake of individuals during 12 months before the examination (18, 19). The consumption frequency of each food item on a daily, weekly or monthly basis was converted to daily intakes; portion sizes were then converted to grams using household measures. Dietary intakes at third examination were considered as dietary intake exposure at baseline.
Two scores were computed to determine the degree of adherence to the MedDiet. The Mediterranean diet score (MDS) was computed as defined by Trichopoulou et al. (20). Briefly, a value of 0 or 1 was assigned to each component using sex-specific median as cut-off. One point was assigned to individuals whose consumption of expected beneficial components (vegetable, legumes, fruits/nuts, cereals and fish) was equal or above the sex specific median and a value of zero was assigned for individuals whose consumption was below the median. For components presumed to be detrimental (meat and dairy products), 1 point was assigned if consumption was below the sex-specific median value and a value of zero otherwise. Because of the low consumption of olive oil in Iran, the ratio of unsaturated fat (monounsaturated plus polyunsaturated) to saturated was calculated for lipid intake; a value of 1 was assigned if this ratio was equal or above the sex specific median value and a value of zero was assigned for consumption below the median (21). Because of religious reasons, alcohol consumption is not usual or probably underreported in the Iranian population. Therefore, alcohol consumption was not considered as a food component. Thus, the total MDS scores ranged from zero (minimal adherence) to 8 (maximal adherence). Each food component was energy adjusted using the energy density method (g/1000 kcal) to determine the MDS score.
Adherence to the MedDiet was also assessed using sex-specific absolute values as the cut-off proposed by Sofi et al. for daily intakes of each of the above-mentioned food components (Sofi-MDS) (22). For each food component, the optimal numbers of change-points and the absolute values for these change-points were proposed. Briefly, values of zero or 2 were assigned to each components with one change point and zero or 1 or 2 to each component with 2 change-points. For presumed beneficial components, a score of 0, 1 or 2 was assigned to the lowest, middle and highest categories of intake. The scoring was inverted for the two presumed detrimental components. For this score, olive oil consumption was considered for lipid intake as a single dichotomized variable (yes/no). This score range was originally 0 to 16, but the possible maximum score in our study was 14, due to not considering alcohol consumption as a food component.
3.2. Other Measurements
Information on demographic variables, smoking status (yes/no), medical history and drug use were obtained using a pre-tested questionnaire. Participants who smoked daily or occasionally were called smokers and those who had never smoked were non-smokers.
Physical activity (PA) during the previous year was evaluated using the modified Kriska’s PA questionnaire to obtain the frequency and the time spent on light, moderate, high and very high intensity activities according to a list of common activity of daily life (23, 24). Physical activity was expressed as metabolic equivalent minute per week (MET-min/wk).
Weight was measured to the nearest 100 g, wearing light clothes and without shoes. Height was measured to the nearest 0.5 cm in a standing position without shoes. WC was measured to the nearest 0.1 cm at the narrowest level over light clothing using an unscratched tape meter. BMI (Body Mass Index) was calculated as weight (Kg) divided by the square of the height (m²). BP was measured on the right arm in a sitting position, after a 15 minutes rest; the average of two such measurements considered as participants’ blood pressure (16).
For biochemical measurements, blood samples were obtained after 12 - 14 hours overnight fasting. FPG and 2 hours-plasma glucose (2 hours-PG) post oral glucose tolerance test were measured by enzymatic colorimetric method with glucose oxidase. TG concentrations were measured by the enzymatic colorimetric method with glycerol phosphate oxidase and cholesterol esterase and cholesterol oxidase, respectively. HDL-C was measured after precipitation of apo-lipoprotein β with phosphotungstic acid. Analyses were performed using Pars Azmoon kits (Pars Azmoon Inc., Tehran, Iran) and a Selectra 2 auto-analyzer (Vital Scientific, Spankeren, The Netherlands). Inter- and intra-assay coefficients of variation (CVs) were 2.2% and 2.2 % for FPG, 0.6% and 1.6% for TG, 0.5% and 2% for TC and HDL-C, respectively (15).
3.3. Metabolic Syndrome Ascertainment
MetS was defined according to the recent interim consensus as having three or more of the following criteria (1): Abdominal obesity (WC ≥ 90 cm in both sexes (25)), high TG (≥ 1.7 mmol/L (150 mg/dL) or drug treatment for high triglycerides), low HDL-C (< 1.0 mmol/L (40 mg/dL) in men or < 1.3 mmol/L (50 mg/dL) in women or drug treatment), high blood pressure (SBP/DBP ≥ 130/85 or antihypertensive drug treatment and high FPG (≥ 5.6 mmol/L (100 mg/dL) or drug treatment for elevated glucose).
3.4. Statistical Analysis
Participants were divided into tertiles according to their scores of adherence to the MedDiet. Baseline characteristics across tertiles of the diet score were compared using ANOVA for continuous variables and chi-square test for categorical variables. Based on existing literature and statistical tests, the following variables were considered as potential confounders: Age (continuous), sex (male/female), smoking (yes/no), physical activity (MET score in tertiles, or missing) at baseline, energy intake (continuous), BMI at baseline and BMI change in the 3-year follow-up. Means/geometric means (95% CI) for MetS components at follow-up examination across tertiles of the diet scores were determined using a general linear model, after adjusting for all the aforementioned confounders. FPG, TG and HDL were log-transformed to improve their normal distribution before analyses and then geometric means were reported. Participants who took any related medication were excluded from these analyses. In addition, odds (95% CI) of abnormalities incidence in each MetS components across tertiles of the diet scores were examined using logistic regression analysis after adjusting for potential confounders. In the analyses for incidence of abnormality in each component, prevalent events at baseline were excluded.
Odds ratio (OR) and 95 % CI of the MetS incidence across tertiles of the diet scores was examined using logistic regression analysis. Two models were constructed: Model 1 was adjusted for age and sex, and model 2 was further adjusted for smoking, physical activity, energy intake, BMI at baseline and BMI change. Tests for trend across dietary pattern scores were performed by assigning the median value of the tertiles to the respective categories and entering this as continuous variable into the models. Interactions between MedDiet scores and sex for the MetS incidence were tested by including cross products in our final model. All statistical analyses were conducted using SPSS (Version 15.0; Chicago, IL, USA) and P-values < 0.05 were considered significant.
4. Results
The mean age of our participants was 39.1 ± 13.6 years at baseline and 55.2% of them were females. The mean MDS was 4.4 (SD 1.5), ranged 0 - 8; 51.5 % had a MDS < 5. The mean Sofi-MDS was 7.3 (SD 2.1), ranged 2 - 14. According to Sofi-MDS, the proportion of the study population that attained the maximum score of 2 was highest for cereals (93.1% and 99.1% in female and male, respectively) and legumes (65.5% and 68.1% in females and males, respectively), and was lowest for vegetables (11.2% and 5.9% in females and males) and fish (31.2% and 24.6% in females and males) among the five beneficial food groups. For dairy products, 10.3% of females and 16.9% males had intakes of the proposed values of < 100 and ≤ 150 g/day, respectively and therefore attained the score of 2. For meat and meat products, 95.3% of females (intake < 130 g) and 93.9% of males (< 140 g) attained the maximum score of 2. Baseline characteristics of participants across tertiles of Mediterranean dietary pattern scores are presented in Table 1. Participants with higher MDS score were older, less often women, had higher BMI and had lower dietary intakes of energy, saturated fatty acids, monounsaturated fatty acid, polyunsaturated fatty acid, calcium and magnesium. Participants with higher Sofi-MDS score were also older, more likely to be women and more active. A higher Sofi-MDS was associated with higher dietary intakes of energy, saturated fatty acids, monounsaturated fatty acid, polyunsaturated fatty acid, fiber, calcium and magnesium.
| Characteristics | MDS | Sofi-MDS | ||||||
|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | P Value b | T1 | T2 | T3 | P Value b | |
| Score cut-off | 0 - 3 | 4 | 5 - 8 | 2 - 6 | 7 - 8 | 9 - 14 | ||
| Number | 586 | 568 | 1087 | 902 | 789 | 550 | ||
| Age, y | 36.3 (13.3) | 37.9 (13.0) | 41.3 (13.8) | < 0.001 | 38.4 (13.6) | 39.1 (13.8) | 40.4 (13.2) | 0.024 |
| Sex (female), % | 65.2 | 56.2 | 49.4 | < 0.001 | 53.8 | 51.5 | 63.6 | < 0.001 |
| Smokers | 11.9 | 17.1 | 14.2 | 0.044 | 15.1 | 14.2 | 13.3 | 0.630 |
| BMI, kg/m2 | 26.5 (5.2) | 26.4 (4.8) | 27.1 (4.8) | 0.006 | 26.5 (4.9) | 26.7 (4.9) | 26.5 (5.0) | < 0.001 |
| Physical activity, Met-min/week c | 0.935 | 0.003 | ||||||
| < 126 | 33.5 | 34.7 | 32.7 | 36.8 | 34.5 | 26.3 | ||
| 126 - 610 | 33.9 | 32.7 | 33.1 | 32.2 | 31.9 | 36.5 | ||
| > 610 | 32.7 | 32.7 | 34.2 | 30.9 | 33.5 | 37.3 | ||
| Energy intake, Kcal/day | 2532 (811) | 2308 (779) | 2164 (705) | < 0.001 | 2065 (716) | 2347 (762) | 2604 (739) | < 0.001 |
| Carbohydrate, % of total energy | 54.4 (7.62) | 57.0 (7.19) | 59.7 (6.58) | 0.001 | 56.3 (7.75) | 58.3 (6.89) | 58.9 (6.94) | < 0.001 |
| Protein, % of total energy | 13.9 (2.74) | 13.6 (2.50) | 13.5 (2.28) | < 0.001 | 13.5 (2.56) | 13.6 (2.41) | 13.9 (2.37) | 0.004 |
| Fat, % of total energy | 33.5 (7.77) | 31.6 (7.17) | 29.8 (6.39) | < 0.001 | 32.3 (7.75) | 30.6 (6.55) | 30.4 (6.71) | < 0.001 |
| Saturated fat, g/d | 34.5 (24.9) | 26.9 (11.0) | 22.6 (9.2) | < 0.001 | 25.7 (20.4) | 26.8 (12.3) | 28.6 (12.1) | 0.004 |
| Monounsaturated fat, g/d | 32.4 (14.6) | 28.0 (12.0) | 25.0 (10.2) | < 0.001 | 25.8 (12.2) | 27.6 (11.8) | 30.9 (12.8) | < 0.001 |
| Polyunsaturated fat, g/d | 18.4 (10.2) | 16.9 (8.50) | 15.7 (7.55) | < 0.001 | 15.6 (8.39) | 16.6 (8.23) | 18.5 (9.19) | < 0.001 |
| Dietary fiber, g/d | 36.4 (23.2) | 38.3 (22.9) | 38.9 (20.5) | 0.076 | 31.7 (21.0) | 39.8 (21.6) | 46.0 (20.6) | < 0.001 |
| Calcium, mg/d | 1432 (666) | 1225 (517) | 1144 (534) | < 0.001 | 1100 (465) | 1245 (563) | 1461 (694) | < 0.001 |
| Magnesium, mg/d | 394 (156) | 374 (146) | 371 (144) | < 0.001 | 325 (130) | 384 (139) | 456 (152) | < 0.001 |
a Abbreviation: MDS, the Mediterranean diet score.
b P-Value based on ANOVA and chi-square test.
c There were 287 missing data on physical activity.
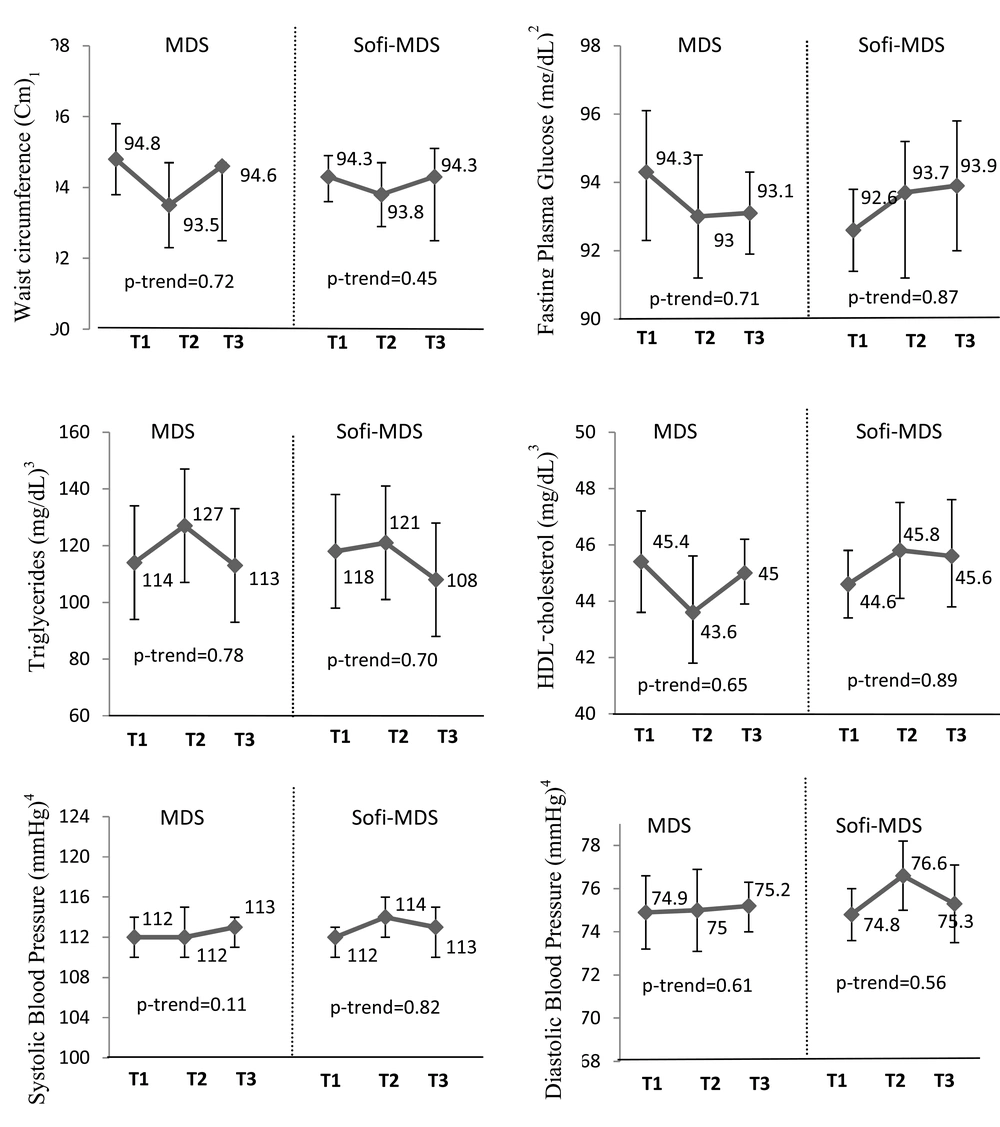
Figure 1 presents the association between the MedDiet scores and MetS components at follow-up across tertiles of the MedDiet scores. After adjusting for potential covariates, no significant differences were observed in the means of MetS components across tertiles of the Mediterranean dietary pattern scores at follow-up examinations (Figure 1). For the five components of MetS, the incidence of abnormalities identified during 3 years follow-up were 337 (30%) for high WC, 327 (16%) for high FPG, 221 (14.4%) for high TG, 96 (11.8%) for low HDL-C and 320 (17.4%) for high BP. There were no significant differences in the odds of 3-year abnormality in each of the MetS components across tertiles of the MedDiet scores (Table 2).
| Mediterranean diet scores | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diet Score | Total | T1 | T2 | T3 | P-trend | ||||||
| n (%) b | OR | 95%CI | n (%) b | OR | 95%CI | n (%) b | OR | 95%CI | |||
| MDS | |||||||||||
| High WCc | 1123 | 103 (30) | 1.00 | ref | 87 (28.8) | 0.86 | 0.54, 1.38 | 147 (30.8) | 0.74 | 0.48, 1.13 | 0.56 |
| High FPGd | 2045 | 74 (13.7) | 1.00 | ref | 82 (15.5) | 1.01 | 0.77, 1.57 | 171 (17.5) | 1.01 | 0.73, 1.39 | 0.55 |
| High TGe | 1530 | 63 (14.4) | 1.00 | ref | 48 (12.3) | 0.74 | 0.48, 1.12 | 110 (15.7) | 0.81 | 0.56, 1.17 | 0.43 |
| Low HDLf | 817 | 32 (14.3) | 1.00 | ref | 25 (12.6) | 0.99 | 0.55, 1.78 | 39 (9.9) | 0.82 | 0.48, 1.40 | 0.22 |
| High BPg | 1837 | 85(17.0) | 1.00 | ref | 65 (13.6) | 0.70 | 0.48,1.02 | 170 (19.8) | 0.89 | 0.64, 1.22 | 0.95 |
| Sofi-MDS | |||||||||||
| High WC | 1123 | 128(26.9) | 1.00 | ref | 108 (29.3) | 1.07 | 0.71, 1.62 | 101 (36.1) | 0.84 | 0.52, 1.33 | 0.82 |
| High FPG | 2045 | 125 (15.1) | 1.00 | ref | 102 (14.2) | 0.82 | 0.61, 1.10 | 100 (20.0) | 1.23 | 0.89, 1.70 | 0.40 |
| High TG | 1530 | 89 (14.2) | 1.00 | ref | 84 (15.8) | 0.95 | 0.67, 1.34 | 48 (13.0) | 0.77 | 0.51, 1.17 | 0.79 |
| Low HDL | 817 | 34 (10.0) | 1.00 | ref | 37 (12.8) | 1.15 | 0.68, 1.96 | 25 (13.2) | 1.09 | 0.60, 1.99 | 0.16 |
| High BP | 1837 | 126 (16.7) | 1.00 | ref | 121 (19.1) | 10.06 | 0.77, 1.45 | 73 (16.2) | 0.82 | 0.56, 1.20 | 0.17 |
a Adjusted for age, sex, energy Intake, physical activity, smoking, BMI baseline and BMI Change in 3 years follow-up. Abbreviations: WC, waist circumference; FPG, fasting plasma glucose; TG, Triglycerides; HDL-C, HDL cholesterol; BP, blood pressure; ref, referent category.
b Number of new cases for each component by tertiles.
c WC ≥ 90 cm for both sexes.
d FPG ≥ 5.6 mmol/L or drug treatment for elevated glucose.
e TG ≥ 1.7 mmol/L or drug treatment for high triglycerides.
f HDL-C (< 1.0 mmol/L in men or < 1.3 mmol/L) in women or drug treatment.
g Systolic ≥ 130 and/or diastolic ≥ 85 mmHg or antihypertensive drug treatment.
Values are adjusted mean/geometric means (95% CI). Values are adjusted for age, sex, energy intake, physical activity, smoking, BMI baseline and BMI change in 3-year follow-up. 1 n = 2241. 2 n = 2216 who were not treated by oral hypoglycemia or insulin medications. 3 n = 2069 who were not treated by cholesterol-lowering medications. 4 n = 2055 who were not treated by hypertension-lowering medications.
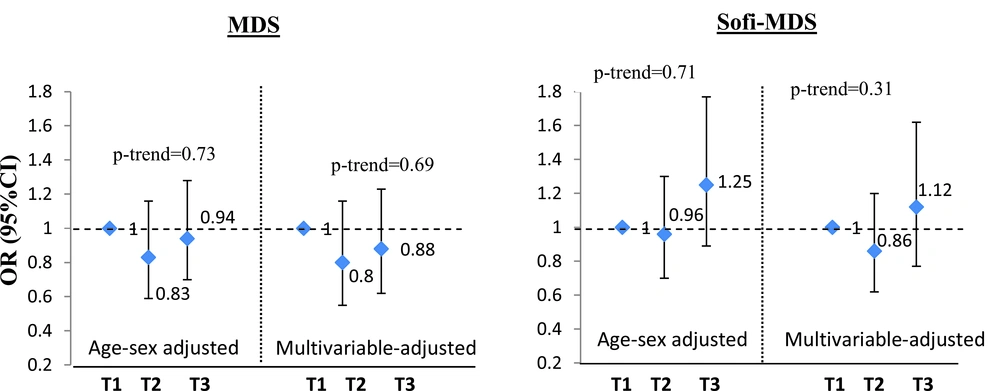
A total of 246 incident MetS cases were identified during the 3-year follow-up among 1661 participants, free of metabolic syndrome at baseline. The adjusted odds ratio (OR) of MetS incidence did not differ significantly in participants with the highest tertile of the MDS or Sofi-MDS compared to those in the lowest categories in both age and sex adjusted model and the multivariable adjusted model (Figure 2). There were no significant interactions by sex in the relations between MDS (P = 0.52) or Sofi-MDS (P = 0.12) and incident MetS.
5. Discussion
In this prospective study conducted on Iranian adults without type 2 diabetes, greater adherence to the MedDiet was not associated with either lower MetS components or lower incidence of metabolic abnormalities, after 3 years of follow-up. In addition, we did not find any significant associations between the two scores used to assess adherence to this pattern and the 3-year MetS incidence, after adjusting for potential confounders.
Previous prospective studies consistently report an inverse association between adherence to the MedDiet and MetS incidence, though the strength of the association was varied (7-9). However, previous findings on the association between MedDiet and MetS components have been contradictory (7-9, 11, 12). In a Spanish population, adherence to the MedDiet according to MDS was related to 80% lower risk of MetS after a 6-year follow-up, but the score was only related to WC (mean difference between the highest and lowest tertiles: 0.5 cm, p-trend: 0.04) (7). In a USA population, participants with higher adherence to the MedDiet according to the Mediterranean style-dietary pattern score (MSDPS) had lowest cumulative incidence of MetS over 7 years of follow-up. In that study, WC, FPG, TG and HDL-C were significantly related to the MSDPS (8). The last prospective study conducted among French adults showed that higher adherence to the MedDiet was associated with 50% and 53% lower MetS incident after 6 years of follow-up based on MDS score and a revised Mediterranean score (MED) respectively, while according to the MSDPS the inverse association became non-significant after adjusting for potential confounders (9). While MSDP was related to most MetS components in a USA population, this index in the French study was related only to HDL-C. However, MED score was associated to WC, SBP, TG and HDL-C (9). Some discrepancies between the studies could be due to the score used to evaluate adherence to the MedDiet that can affect strength and the significance of the associations (6, 26). A previous prospective study conducted in the TLGS also found no significant association between MDS and WC after 6.7 years of follow-up (27).
Different indexes were developed to assess the adherence to the MedDiet with varying in the components included, the weight given to each component and the scoring system used (26). Using MDS as suggested by Trichopoulou et al. (20) with some modification, 48.5% of our participants had the highest degree of adherence (Tertile 3). Since the scoring system for calculating MDS is based on sample-specific median consumption, we considered the absolute values for each component as proposed by Sofi et al. in the second scoring of the MedDiet (22). According to Sofi et al., the actual amounts for each food component should be consumed to describe adherence to the MedDiet (22). Based on the score, only 24.5% of our participants had the highest adherence. Adherence to the MedDiet increased with age in both the scores used. Intakes of energy, macronutrients and nutrients across the tertiles of the diet scores were different, because calculation of the first score was based on energy-adjusted median of each component, while for Sofi-MDS, absolute values were used. None of these two scores was associated with MetS and its components in our study, which could suggest that something beyond quantitative differences in the intakes of each component in our population, compared to the Mediterranean countries, may lead to non-significant associations observed in our study between the pattern and MetS.
Cereals as a beneficial food item include both refined and whole cereals in determination of the degree adherence to the MedDiet, although in the traditional MedDiet, cereals were largely unrefined. The effects of refined- and whole-cereals on health may be distinct and this is one of the concerns about using the MedDiet scores (28, 29). More than 90% of our population met the proposed values intake of cereals according to the Sofi-MDS. However, in an Iranian population, white rice and refined cereals constituted the major part of daily cereals consumption. Previous studies on the association between white rice and MetS provided inconsistent findings despite most investigations suggesting an increased risk of developing diabetes and cardio metabolic risk factors with increasing rice consumption (30). However, the non-significant association of white rice consumption and the risk of MetS in Iranian participants on high-fiber diet suggest that the possible adverse effects of white rice on metabolic outcomes could be, in part, due to low consumption of fruit and vegetable intake (30).
The daily median intake of fish in our population was lower than the median intake in a Greek population (6.4 vs. 18.8 g/day for female; 7.1 vs. 23.7 g/day for male). According to Sofi-MDS, 31.2% of women and 24.6% of men consumed the highest value intake of fish and seafood. In addition to variation in quantity of fish intake, differences in the type of fish consumed and the way of cooking between the Mediterranean and non-Mediterranean countries can affect the amount of n-3 PUFA intake (10). In Mediterranean countries, frying fish with olive oil, especially virgin olive oil, is the most common way of preparing fish, which has been found to increase the nutritional benefits of fish because of the absorption of antioxidants phenolics, terpenic acids and vitamin E. In Iran, frying fish with sunflower or corn oil is the most common method of preparing fish, which has been shown to reduce n-3 and increase n-6 fatty acid content of fish (10).
While using olive oil for cooking and salad dressing daily is common in the Mediterranean countries, few people in Iran consume olive oil. Therefore, we considered the ratio of unsaturated fat to SFA instead of median intake of olive oil in the first score (21). However, because of high consumption of other vegetable oils, including sunflower and corn oils for cooking in Iran, there is higher consumption of PUFA, especially n-6 than n-3 PUFA and MUFA. According to the second score, Sofi-MDS, only 4.8% of our participants were categorized as daily users of olive oil and those whose intake of olive oil was still low with a mean consumption of 18 g/day. Therefore, most of MUFA intake in our populations is from animal fat rather than olive oil. Olive oil, especially virgin olive oil, as a key component of the MedDiet has a fundamental effect on prevention of chronic diseases because of not only high content of MUFA, but also existence of its nonsaponifiable fraction (10).
It has been well explained by Hoffman and Gerber that nutritional benefits or detriments that different populations receive from consuming each component of the MedDiet can vary and cannot be assessed simply by absolute levels of their consumptions (10). Differences in availability of foods, preferences for eating of foods in each food groups, processing and preparation of foods that influence the composition of a food between the Mediterranean and non-Mediterranean countries can also affect the overall health benefits of the MedDiet in non-Mediterranean countries (10). Most extensive epidemiological evidence supporting the beneficial effects of the MedDiet has been documented in the Mediterranean countries (5, 6). In addition, according to the results of a meta-analysis, the effect of the MedDiet on metabolic syndrome and its components has been more prominent in Mediterranean countries (5). Therefore, it seems that the protective effect of the MedDiet against chronic diseases could be attributed to the diet and other eating behaviors such as the time of eating, the order of courses in each meal and the meal patterns or other potentially confounders such as genetics and sun exposure (10, 31).
In this study, we adjusted for BMI change over 3 years follow-up to control the confounding effect of change in BMI on the associations between MedDiet and MetS incidence. However, BMI change is likely to mediate the effect of MedDiet on MetS incidence. Some previous studies suggested an inverse association between MedDiet and the risk of MetS independent of BMI and BMI change over time (8, 9). However, in this study we could not find any significant associations before (age and sex adjusted model) and after adjustment for BMI change.
Prospective design, using recently proposed index considering the absolute amount of each food components in addition to calculating the score according to the sex-specific median intake to ascertain conformity to the MedDiet, and using an FFQ specially developed and validated in our population are strengths of our study. However, our study had some limitations that may explain lack of association. First, scoring participants according to the MedDiet in an Iranian population might be inefficient, because the dietary patterns are very different from those living in Mediterranean countries. Another limitation was possible concerns about using an FFQ to measure food consumption, which may increase the possibility of overestimation in consumption of healthy food items and underestimation in consumption of unhealthy food items (31).
In conclusion, we could not find any significant associations between adherence to the MedDiet, according to MDS and Sofi-MDS and MetS components and MetS incidence after 3 years of follow-up in an Iranian population. More studies in non-Mediterranean countries are needed to investigate the applicability of the MedDiet and its benefits to the prevention of metabolic abnormalities.