1. Background
Primary aldosteronism (PA) has been traditionally considered a rare cause of hypertension (1). However, recent studies have shown a higher prevalence, ranging between 5% - 20 % among hypertensive patients (2). Moreover, recent studies have demonstrated a higher frequency of cardiovascular complications in PA compared to patients with essential hypertension (3, 4). However, the mechanisms by which excess aldosterone induces cardiovascular damage remain undetermined. Endothelial dysfunction could play a role in the deleterious cardiovascular effects of aldosterone, as it predicts long-term atherosclerotic disease progression and the risk of cardiovascular events (5, 6). Asymmetric dimethylarginine (ADMA) is one of the most widely used and validated markers for evaluating the endothelial function. ADMA is described as an inhibitor of nitric oxide synthase (7). Elevated plasma ADMA has been identified as a predictor of acute coronary events and an independent risk factor for all-cause and cardiovascular mortality (8, 9). Several studies have reported elevated AMDA concentration in patients with different cardiovascular and metabolic disorders as essential hypertension (10), diabetes mellitus (11), hypercholesterolemia (12), coronary artery disease (13), but reports on ADMA levels in endocrine hypertension are scarce.
Increased plasma concentrations of ADMA could contribute to endothelial dysfunction, increased atherogenesis and elevated cardiovascular risk in patients with PA.
2. Objectives
The aim of this study was to compare the levels of ADMA among patients with PA, controls with essential hypertension (EH) and healthy participants.
3. Methods
3.1. Study Population
The study population consisted of 18 patients with PA, 18 controls with EH, and 18 healthy participants. All studies were performed after obtaining a written informed consent form according to a protocol approved by the ethics committee of Sofia Medical University. Hypertensive patients were referred to the clinical center of endocrinology in Sofia for diagnosis and treatment during June 2009 - June 2011. In patients with EH other secondary forms of hypertension were ruled out, using medical history, physical examination, biochemical and hormonal investigations and imaging studies. The diagnosis of PA was established, using the following algorithm: Medications that can interfere with aldosterone and renin measurements (beta-blockers, diuretics, angiotensin II receptor blockers, angiotensin-converting enzyme inhibitors) were discontinued for at least 7 - 10 days, and spironolactone was stopped for at least 45 days prior to blood sampling. Centrally acting medicaments (Moxonodine, Rilmenidine), alpha-blockers, and calcium channel blockers were used to treat hypertension. Blood samples for plasma renin activity (PRA) and aldosterone were taken in the morning between 8 - 10 hours a.m. after the patient had been in a sitting position for 30 minutes. The ARR was then calculated and a confirmatory test, Captopril test, was performed in patients with high ARR (above 750 pmol/L per ng/mL/h) (10) and aldosterone above 416 pmol/L. The diagnosis of PA was considered if the ARR was > 970 after the oral administration of 50 mg of Captopril, while the patient has been sitting for 90 minutes (14).
3.2. Laboratory Assays
Plasma renin activity (ng/mL/h) was determined by quantitative determination of angiotensin I, using a commercially available radioimmunoassay kit (DiaSorin S.p.A., Saluggia (VC), Italy). Serum aldosterone was measured by radioimmunoassay (Immunotech, Beckman Coulter Company, Marseille, France) and expressed in picomols per liter (pmol/L). The analytical sensitivity of the method was 0.20 ng/ml, inter-assay CV 7.5%, respectively, and intra-assay CV 5.4%. The cross reactivity with heptapeptide, angiotensin II, and hexapeptide was below 0.02%. We elaborated our own reference range for PRA in a sitting position (0.3-3.5 ng/mL/h) (15). In cases in which PRA was < 0.3 ng/mL/h or undetectable, it was set at 0.3 ng/mL/h. Serum aldosterone was measured by RIA (Immunotech, Beckman Coulter). For intra-assay assessment, the samples were analyzed 20 times in the same series. The CV was found below 12.6%. For inter-assay assessment, the samples were assayed in duplicate in 10 different series. The CV was found to be < 17.2%. The analytical and functional sensitivity were 1.44 pg/mL, and 6.98 pg/mL, respectively. According to the manufacturer antibody used in this immunoassay was highly specific for aldosterone. Extremely low cross reactivities were found against other naturally occurring steroids (cortisone, corticosterone, DHEAs, etc…) Serum ADMA levels were determined, using competitive enzyme-linked immunosorbent assay (ELISA) (Human ADMA ELISA kit, PromoKine, Heidelberg, Germany; Thermo electron corporation: APPLISCAN). The analytical sensitivity of this method was 0.05 µmol/L and cross-reactivity with SDMA - < 0.5. Intra-assay and inter-assay CV for ADMA were 4.1 % and 4.5 %, respectively. Glucose was measured, using enzymatic reference method with hexokinase (Cobas Integra, Roche diagnostics). Intra-assay and inter-assay CV for glucose were 1.7% and 2.6% at 5.3 mmol/L, respectively. Serum lipid parameters were assessed, using an enzymatic colorimetric method (Cobas Integra, Roche diagnostics). Intra-assay and inter-assay CV for total cholesterol were 1.6% and 1.6%, respectively. Intra-assay and inter-assay CV for triglycerides were 1.6% and 3.7%, respectively. Intra-assay and inter-assay CV for HDL- cholesterol were 2.5 and 2.7%, respectively.
The inter-assay (QC Low and QC High) was calculated, using mean analytic value of all plates according to the formula: CV (%) = standard deviation / mean value of analytic * 100, which corresponds to IFCC protocol. According to generally accepted quality criteria cited above, CV% for inter- and intra-assay was acceptable, which ensured the reliability of the results.
3.3. Statistical Analysis
Statistical analysis was performed using SPSS 17.0 for Windows (Chicago, Illinois, USA). Statistical significance was set at 0.05. Data distribution normality was tested with Kolmogorov-Smirnov test. Gaussian-distributed data are shown as mean ± SD. Student’s t-test for independent samples was used to compare the equality of means for the two groups, and ANOVA was used when testing three groups. Categorical data were compared by χ2 test. The relationship between the two metrical parameters was investigated by Pearson correlation.
4. Results
The baseline demographic and metabolic characteristics of those patients with primary aldosteronism, essential hypertension and normotensive controls are demonstrated in Table 1. No significant differences were observed among the three groups in age, gender and fasting blood glucose. Patients with PA had higher body mass index and triglycerides and lower high- density (HDL) lipoprotein cholesterol compared to normotensive controls (P = 0.001, 0.016, 0.002, respectively). Patients with EH had higher levels of total cholesterol compared to patients with PA (P = 0.024). Patients with EH presented with significantly higher total cholesterol, low- density lipoprotein (LDL) cholesterol and triglycerides and lower HDL cholesterol than the control group (P = 0.025, P = 0.012, P = 0.012 and P = 0.011, respectively).
| Variable | PA, n = 18 | EH, n = 18 | HC, n = 18 | P Value |
|---|---|---|---|---|
| Age at presentation, y | 56.7 ± 9.6 | 52.6 ± 9.7 | 50.7 ± 13.5 | 0.258 |
| Men, (%) | 6 (33.3) | 9 (50) | 9 (50) | 0.509 |
| BMI, kg/m² | 29.0 ± 5.8 | 27.1 ± 3.8 | 23.2 ± 2.7 | 0.001 |
| Fasting blood glucose, mmol/L | 5.8 ± 1.6 | 4.8 ± 1.3 | 5.2 ± 0.7 | 0.076 |
| Total cholesterol, mmol/L | 5.1 ± 0.8 | 5.9 ± 1.2 | 5.2 ± 0.9 | 0.029 |
| Triglycerides, mmol/L | 1.3 ± 0.4 | 1.6 ± 0.9 | 0.9 ± 0.5 | 0.017 |
| HDL cholesterol, mmol/L | 1.2 ± 0.4 | 1.3 ± 0.3 | 1.6 ± 0.4 | 0.002 |
| LDL cholesterol mmol/L | 3.3 ± 0.8 | 3.9 ± 1.2 | 3.1 ± 0.8 | 0.041 |
Abbreviations: BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
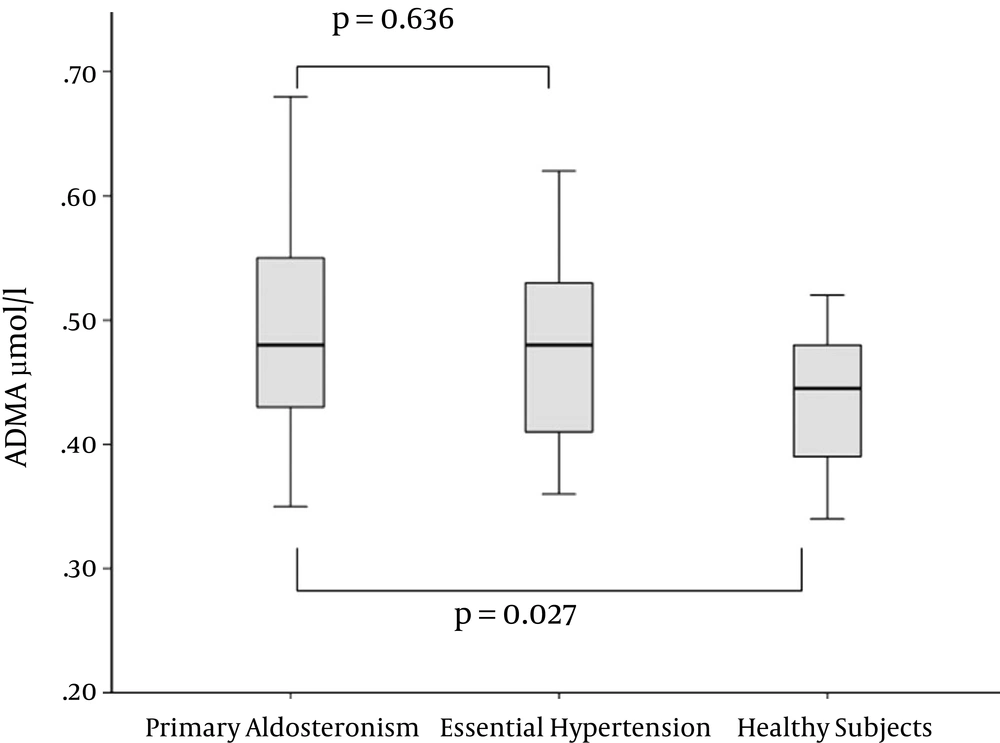
ADMA levels were compared in patients with PA, EH and healthy controls (Figure 1). Concentrations of ADMA were significantly higher in patients with PA than in healthy controls (0.49 ± 0.09 vs. 0.43 ± 0.05 μmol/L, Р = 0.027). There was no difference in ADMA levels between cases with PA and EH (0.49 ± 0.09 vs. 0.48 ± 0.08 μmol/L, Р = 0.636). The difference between participants with EH and the normotensive control group did not reach statistical significance (P = 0.06).
No significant correlations were found between ADMA and some metabolic parameters in patients with PA (Table 2).
| ADMA | ||
|---|---|---|
| Pearson Coefficient | P Value | |
| ADMA | - | - |
| Age | 0.272 | 0.275 |
| BMI | 0.226 | 0.367 |
| Fasting blood glucose | -0.056 | 0.830 |
| Total cholesterol | 0.006 | 0.980 |
| HDL cholesterol | 0.182 | 0.499 |
| LDL cholesterol | -0.077 | 0.770 |
| Triglycerides | -0.011 | 0.968 |
Abbreviations: ADMA, asymmetric dimethylarginine; BMI, body mass index; HDL, high- density lipoprotein; LDL, low-density lipoprotein.
5. Discussion
Endothelial dysfunction (ED) is characterized with a tendency to proinflammatory and prothrombotic state and reduced vasodilatation. It is now well established that ED is related to the development of atherosclerosis (16), myocardial infarction (17), congestive heart failure (18), diabetes mellitus (19), and peripheral arterial diseases (20). One of the possible mechanisms leading to ED is decreased nitric oxide (NO) bioavailability, which can be caused by decreased NO synthesis. With regards to this mechanism, ADMA, which is an endogenous analogue of L-arginine, was firstly recognized in 1992 as a naturally occurring inhibitor of NO synthase (21). Moreover, elevated plasma ADMA concentration has been identified as a predictor of acute coronary events, and an independent risk factor for all-cause and cardiovascular mortality (8, 9). Although elevated concentrations of ADMA have been found in several cardiovascular and metabolic diseases (10-13, 22-24), to our knowledge, to date, there have been no reports on ADMA levels in cases with PA. Recent studies have demonstrated a higher frequency of cardiovascular events in PA compared to patients with EH (3, 4). However, the mechanisms by which excess aldosterone induces cardiovascular damage remain undetermined. Recent studies have demonstrated that aldosterone impairs endothelial function by suppression of the synthesis of NO (25-27). Experimental studies reported that the cytokine stimulated NO synthesis in vascular smooth muscle cells is inhibited by aldosterone in a dose dependent manner (25). Recently, Nishizaka et al. (26) reported a significant association between aldosterone and impaired endothelial function in human subjects, measured by flow-mediated arterial vasodilatation. In view of these findings, we hypothesized that increased ADMA levels in PA could contribute to the impaired endothelial cell-dependent vasodilator responses. Our results confirmed the possible role of ADMA as a cardiovascular risk marker in PA since patients with PA had higher ADMA levels than healthy controls. On the other hand, numerous reports demonstrate that ADMA concentration is increased in patients with EH compared to healthy individuals (10). The latter findings raise the following question: Is high ADMA concentration in PA related to the effect of aldosterone or to hypertension per se? The comparable levels of ADMA in PA and EH suggest the responsible factor for ADMA dysregulation is hypertension itself rather than the specific aldosterone action. Indeed, several hypotheses for the elevation of ADMA in EH have been formulated, although the mechanism remains unclear. Osanai T et al. (28) have shown that ADMA release is enhanced by shear stress via activation of the NF-kappaB pathway. In addition, it is possible that the oxidative stress, a well- known feature of EH, induces elevated ADMA by suppressing the activity of its metabolizing enzyme-dimethylaminohydrolase (29). Another possibility for the elevated ADMA concentration in PA and EH in our study was the increased LDL cholesterol and triglyceride levels, since it has been shown that LDL cholesterol decreases the activity of dimethylaminohydrolase. On the other hand, we did not find any statistically significant correlation between ADMA and metabolic lipid parameters, which confirms the possibility, that hypertension, itself, rather than the deteriorated lipid profile, results in higher ADMA levels (Table 2). Similar results were found in another study that we published recently, comparing ADMA in patients with pheochromocytoma and the control group of the present study (30). Endothelial function was impaired in pheochromocytoma patients, as shown by the elevated circulating levels of ADMA. The lack of association of these markers with glucose, cateholamines and lipid abnormalities as well as their comparable levels in patients with EH suggests that endothelial dysfunction may be related to hypertension itself.
5.1. Limitations and Strength of the Study
This was the first report to investigate the ADMA levels in PA and provide insight into the possible mechanisms of cardiovascular damage in PA, which is the main strength of this study. Small sample size was the main study limitation, which was due to the restricted number of patients with PA.
5.2. Conclusions
ADMA is an endogenous analogue of L-arginine and a naturally occurring inhibitor of NO synthase. This was the first study to show elevated ADMA levels in patients with PA compared to normotensive controls. Our data raise the possibility for a role of ADMA dysregulation in the cardiovascular damage in PA. On the other hand, ADMA levels were similar in patients with PA and EH, which suggests that endothelial dysfunction is more likely related to hypertension per se, than to the specific etiology of elevated blood pressure.