1. Context
Obesity is characterized by an excessive accumulation of fat in the adipose tissue (1, 2). A product of the obese (Ob) gene, called leptin, is released primarily from adipocytes and plays a key role in regulating body weight (3). In most obese subjects, leptin fails to perform its physiological functions in spite of its high serum levels (4, 5).
Obesity is linked with several disorders including cardiovascular diseases (CVDs), certain types of cancer (6), type 2 diabetes, and it raises the risk of pulmonary defects (7).
Recently, Arteaga-Solis et al. demonstrated that leptin resistance leads to increased parasympathetic tone, which in turn causes bronchoconstriction and obesity-associated asthma (8). Another previous study described a link between hypoventilation and adiposity (9). Phipps et al. proposed that hyperleptinemia is a cause of respiratory failure in obese leptin-resistant subjects (10). It has been suggested that a derangement of leptin expression in adipocytes may affect the lungs and promote asthma (11). Interestingly, a previous study reported the highest levels of serum leptin occur in asthmatic obese patients compared to the other human groups including non-obese controls, obese subjects, and asthmatic non obese patients (12). Serum leptin elevation has already been shown to be an established marker of leptin resistance in severe obesity (4). This might lead to the creation of an imbalance between α-1 antitrypsin (A1AT) and NE activity (13, 14). A severe deficiency of serum A1AT is important in the development of chronic obstructive pulmonary disorder (COPD) and asthma, which might be due to the degradation of lung tissue elastin by NE-enhanced activity (15, 16). Briefly, on the basis of the facts described above, it could be postulated that leptin resistance with a protease-antiprotease imbalance may be vital for the obesity predisposition to pulmonary disorders including, among others, asthma and COPD.
This review is divided into two parts. The first part highlights body weight regulation and the disruption of leptin’s physiological function in obesity. The second part summarizes a possible link between obesity and pulmonary disorders.
2. Evidence Acquisition
2.1. Body Weight Regulation by the Leptin Hormone
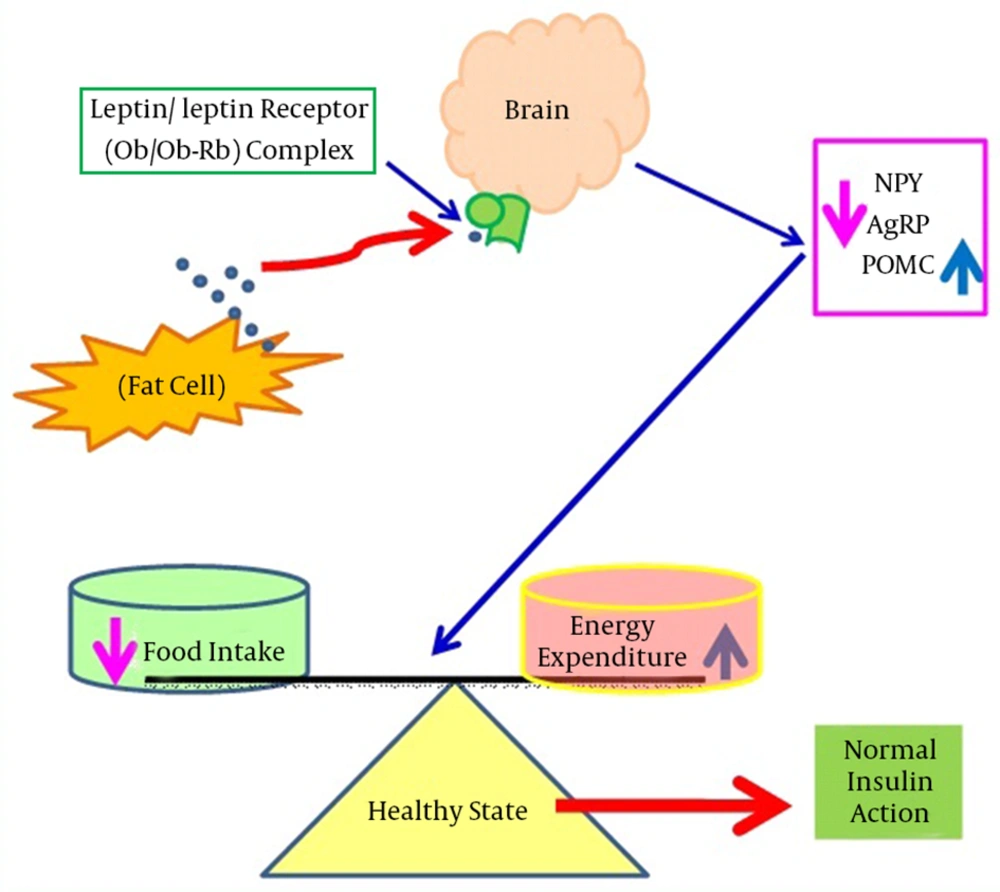
Leptin was first identified in 1994 in the obese (ob/ob) mouse model. It is a 16 kDa non glycated protein consisting of 167 amino acids and is primarily expressed in adipose tissues (17). It is encoded by the obese gene (Ob gene), located on chromosome number 7 in humans, and is responsible for regulating the balance between food intake and energy expenditure (18). During starvation, leptin levels go down, which increases appetite and decreases energy consumption. On the other hand, with sufficient energy stores, leptin inhibits appetite and permits the utilization of energy stores (19). Leptin regulates energy expenditure and food intake by communicating with the central nervous system (CNS) via its receptor (Ob-Rb) located in the hypothalamus (20). The hypothalamus is the key site for leptin detection as it contains two types of neurons: type 1 expresses appetite suppressing peptides derived from pro-opiomelanocortin (POMC) precursors, whereas type 2 produces appetite stimulating peptides such as neuropeptide Y (NPY) and agouti-related peptide (AgRP). Leptin suppresses appetite by counteracting NPY and AgRP, whereas leptin activates POMC mRNA expression, which enhances the release of a potent appetite-suppressing peptide, alpha-melanocyte stimulating hormone (α-MSH) (21, 22). Mechanisms of leptin action and dysfunction are shown in Figures 1 and 2, respectively.
This shows shows that during normal physiological states, leptin binds with its receptors in the brain and suppresses appetite by counteracting NPY and AgRP, however, leptin also induces POMC mRNA expression. Abbreviations: AgRP, agouti-related peptide; mRNA, messenger ribonucleic acid; NPY, neuropeptide Y; Ob, leptin; Ob-Rb, leptin receptor; POMC, pro-opiomelanocortin.
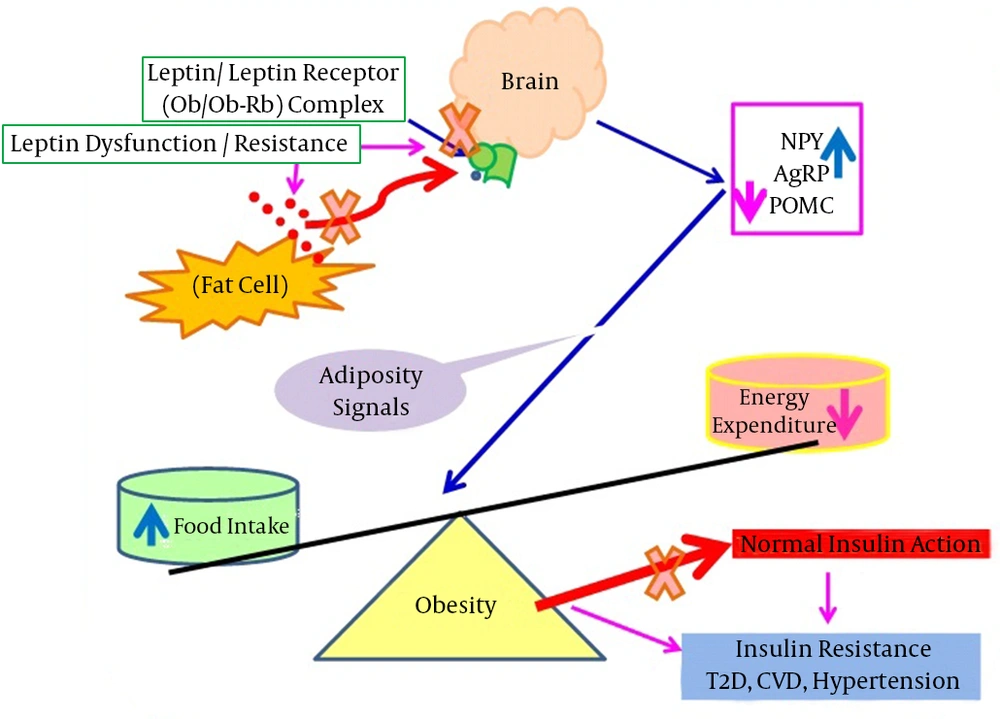
This figure shows that in obesity, leptin cannot bind with its receptors situated in the brain (hypothalamus), resulting in adiposity signals arrived due to the stimulation of NPY and AgRP expression with a concomitant decrease of POMC mRNA expression. The leptin failure leads to severe obesity that is associated with various disorders including insulin resistance, T2D, CVD, hypertension, and asthma. Abbreviations: AgRP, agouti-related peptide; CVD, cardiovascular disease; NPY, neuropeptide Y; Ob, leptin; Ob-Rb, leptin receptor; POMC, pro-opiomelanocortin; T2D, type 2 diabetes.
2.2. Obesity and Leptin Dysfunction
Different causes underlie the disruption of leptin’s physiological functions. Some studies have shown that obese (Ob/Ob) mice are leptin deficient, and diabetic (db/db) mice have mutated leptin receptors contributing to leptin dysfunctions (23, 24). A rare genetic disorder has been reported in obese humans, which might be due to a mutation in the leptin gene and can be treated with exogenous leptin administration. It has been identified that 12 Pakistani, 5 Turkish, 1 Austrian, and 2 Egyptian obese subjects are leptin deficient because of leptin gene mutations (25). On the other hand, several previous studies have reported that most obese individuals have elevated serum leptin levels, which are positively correlated with body mass index (BMI) (26, 27). Despite having increased serum leptin levels, leptin fails to retain its appetite-suppressing effect of reducing weight in obese subjects. Approximately 30 fold higher leptin concentrations were required for weight reduction in obesity (28). The failure of leptin to function in severely obese subjects may be due to extracellular circulating factors. An interaction between circulating leptin and serum leptin interacting proteins (SLIPs) contributes to leptin failures (4, 29). This leptin appetite suppressing effect, which is markedly impaired in obese subjects, is shown in Figure 2.
2.3. Induction of A1AT Expression by Leptin in Hepatocytes
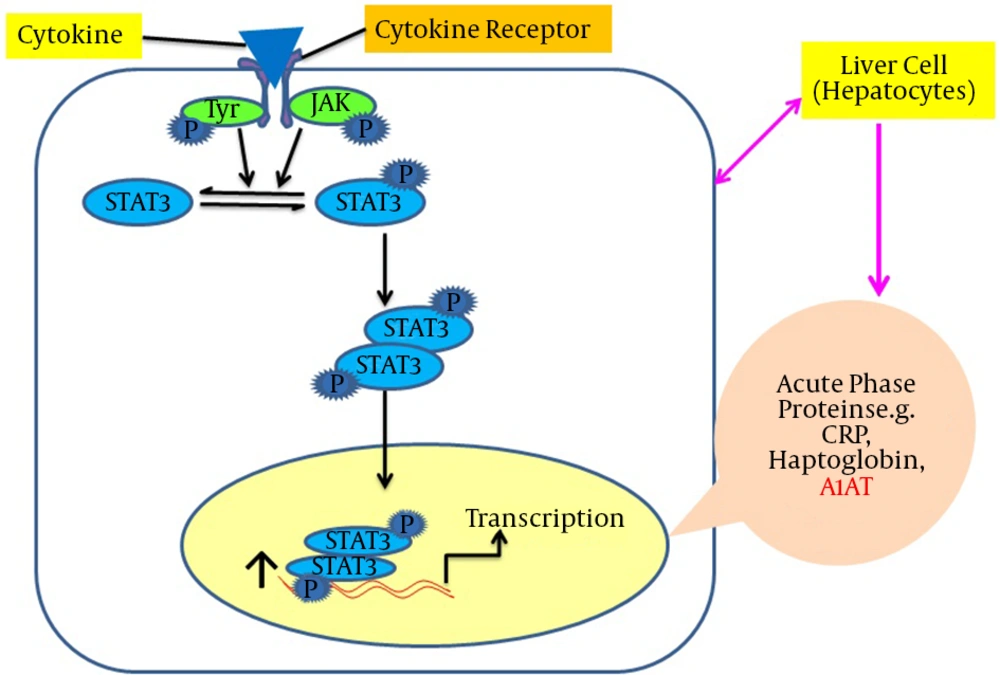
Leptin is structurally identical to the granulocyte colony-stimulating factor (GCSF). GCSF is a member of the interleukin-6 (IL-6) family that includes IL-6 and oncostatin-M (OSM) cytokines (30, 31). In addition to leptin’s control of energy homeostasis by stimulating its hypothalamic receptor, the Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling is essential in peripheral tissues, especially in the liver. This is vital because it affects differential expression of the target genes of acute phase proteins (APPs), including A1AT (13). Using a wide range of techniques, it has been shown that OSM induces a functional response after 24 hours via STAT3 binding to the STAT sequence (32). Moreover, different reports using gel shift and luciferase assays have identified that a perfect consensus for STAT present in the 3'-A1AT enhancer region was capable of binding the transcription factor STAT3 (33). For other cell types such as monocytes and macrophages, lipopolysaccharides (LPS) up-regulate the A1AT gene, which is also stimulated in the lung epithelial tissues in response to OSM (34, 35). In HepG2 cells, the A1AT gene regulation occurs at the transcriptional stage, which is mediated primarily via hepatocyte promoters containing the STAT3 sequence (32). The A1AT levels were stimulated up to three folds by two IL-6 and OSM in HepG2 cell lines (35). It was suggested that the short and long leptin receptor isoforms were expressed in HepG2 hepatic cells, which supports the hypothesis that, like OSM cytokines, leptin might act to regulate few gene targets in hepatocytes (36). Recently, Jiang’s laboratory demonstrated that the expression of the A1AT gene was leptin-dependent in mouse models and leptin stimulation increases the A1AT levels both at the mRNA and protein levels via the JAK/STAT3 pathway in cultured hepatic Hep1-6 cell lines (13). A specific tyrosine residue, Tyr 1138, in the intracellular domain of the leptin receptor (Ob-Rb) mediates the activation of STAT3 (37). The binding of the leptin-ligand causes the Ob-Rb to undergo homo-oligomerization and subsequently binds to JAK2. Only Ob-Rb possesses the STAT-binding site. In vivo studies have demonstrated that STAT3 is the major transcriptional factor in signaling. Binding of Ob-Rb with JAK2 leads to the JAK2 autophosphorylation and the phosphorylation of Tyr985, tyr1077, and Tyr 1138 on the Ob-Rb receptor. The phosphorylation of Tyr1138 recruits STAT3 proteins to the Ob-Rb-JAK2 complex. Tyrosine-phosphorylated STAT3 molecules can dimerize and translocate into the nucleus to activate the transcription of target genes in peripheral tissues such as vascular endothelial cells and HepG2 liver cells (35, 38). An induction of A1AT expression by leptin is illustrated in Figure 3.
This shows shows that leptin binds with its receptors on hepatocytes, successively STAT3 molecules dimerize and translocate into the nucleus and bind with the promoter region of the serpin gene in order to up-regulate the acute phase protein expression, including A1AT. Abbreviations: A1AT, alpha-1-antitrypsin; Jak-STAT3, Janus kinase–signal transducer and activator of transcription.
3. Results
In obesity, the disruption of leptin-mediated signaling occurs that may lead to lung function failure by concomitant increases in body weight. Olson et al. suggested that leptin stimulates lung ventilation, whereas leptin deficiencies lead to hypoventilation in obese subjects (11). The relationship between obesity and asthma is complex and involves several mechanisms (9). A few recent studies have indicated the highest levels of leptin occur in the sera of asthmatic obese patients relative to the other groups such as asthmatic non obese patients, normal obese subjects, and non-obese subjects without asthma or other pulmonary complications. Canoz et al. report 14.5, 40, and 76% increases in serum leptin in asthmatic obese patients compared to obese subjects without asthma and non-obese subjects, with and without asthma, respectively (12). Wahab and colleagues have indicated significant increases in serum leptin levels in obese asthmatic patients (25.8 ± 11.1 ng/mL) compared with non-obese asthmatic patients (8.8 ± 11.1 ng/mL) (39). Another study has shown the maximum serum leptin levels in obese asthmatic patients (19.37 ± 14.04 ng/mL) is elevated compared with non-obese asthmatic patients (6.37 ± 2.46 ng/mL) and healthy controls (6.50 ± 3.51 ng/mL) (40). Mahmoud et al. demonstrated maximum serum leptin levels in COPD cases, both in incitement and static conditions, relative to the other groups. Their calculations indicated that, compared to the non obese control subjects, there were 90.34, 68.75, and 64% increases in serum leptin levels in obese with COPD, obese without COPD, and non-obese with COPD cases, respectively (41). Therefore, these previous investigations clearly indicate that leptin resistance is linked to respiratory/pulmonary-related complications and may contribute to the development of a unique asthma phenotype in obese patients.
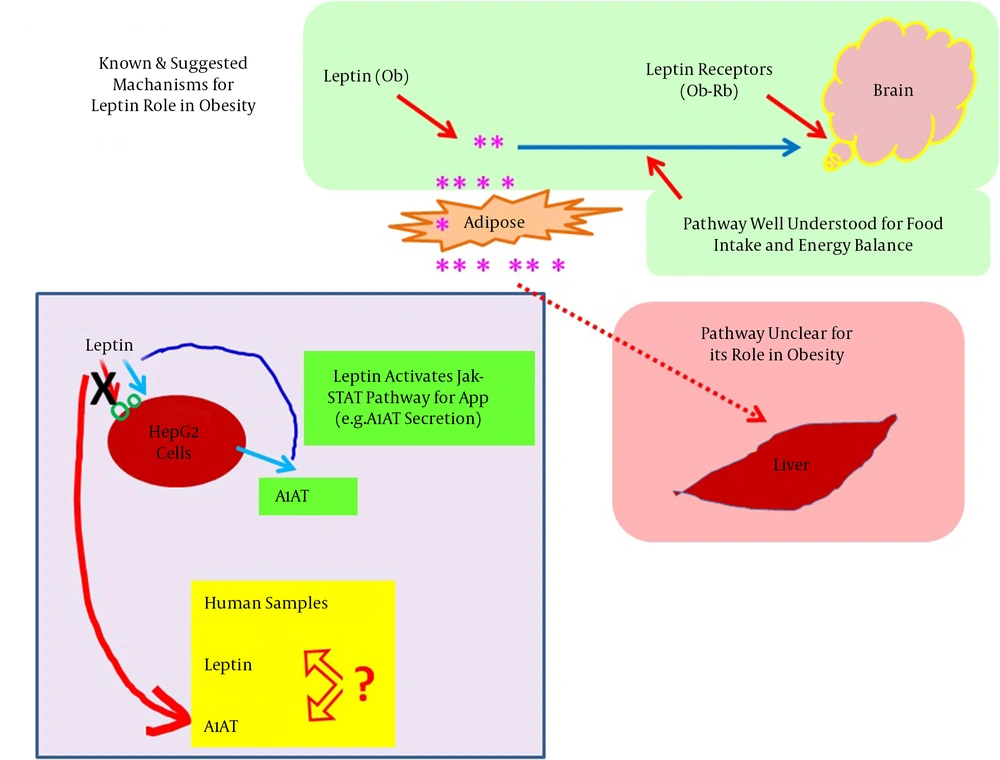
An elegant study reported that leptin stimulates A1AT expression at both the mRNA and protein levels via the JAK2-STAT3 pathway in liver HepG 2 and Hep 1-6 cell lines. Alternatively, serum levels of A1AT are reduced in obese leptin-resistant subjects with parallel increases in neutrophil elastase (NE) activity (13). Elevated serum NE levels are also related to airway constriction in obese subjects (42). In the same obese subjects, increases in C-reactive protein (CRP) have also been reported (42), which induce leptin resistance via CRP-leptin adduct formation (29). An imbalance of A1AT and NE leads to lung tissue impairments (14, 43). Leptin’s actions on the brain and hepatocytes are summarized in Figure 4.
4. Conclusions
4.1. Hypothesis and Outlook for Further Research
In short, previously published data raise the possibility that the protease-antiprotease balance is leptin dependent, and due to the leptin resistance protective capacity of A1AT, it could be arrested to counteract NE activity. Consequently, the degradation of lung tissues, especially elastin, occurs by NE overactivity, which could lead to pulmonary related problems in the susceptible obese population. This hypothesis is summarized as: leptin resistance in asthmatic obese patients may reduce serum alpha 1 antitrypsin levels. In turn, NE enhances, which can lead to the development of pulmonary-related complications including asthma and COPD due to the degradation of proteins in lung tissues.
Exploring mechanisms underlying the derangement of protease antiprotease counterparts will aid in devising personalized interventional therapy for particular obese patients prone to lung complications. This option is preferred over generalizing treatment for all patients suffering from respiratory complications, including non-obese subjects.